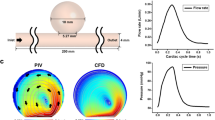
Abstract
Purpose
Pediatric and adult patients with sickle cell anemia (SCA) are at increased risk of stroke and cerebrovascular accident. In the general adult population, there is a relationship between arterial hemodynamics and pathology; however, this relationship in SCA patients remains to be elucidated. The aim of this work was to characterize circle of Willis hemodynamics in patients with SCA and quantify the impact of viscosity choice on pathophysiologically-relevant hemodynamics measures.
Methods
Based on measured vascular geometries, time-varying flow rates, and blood parameters, detailed patient-specific simulations of the circle of Willis were conducted for SCA patients (n = 6). Simulations quantified the impact of patient-specific and standard blood viscosities on wall shear stress (WSS).
Results
These results demonstrated that use of a standard blood viscosity introduces large errors into the estimation of pathophysiologically-relevant hemodynamic parameters. Standard viscosity models overpredicted peak WSS by 55% and 49% for steady and pulsatile flow, respectively. Moreover, these results demonstrated non-uniform, spatial patterns of positive and negative WSS errors related to viscosity, and standard viscosity simulations overpredicted the time-averaged WSS by 32% (standard deviation = 7.1%). Finally, differences in shear rate demonstrated that the viscosity choice alters the simulated near-wall flow field, impacting hemodynamics measures.
Conclusions
This work presents simulations of circle of Willis arterial flow in SCA patients and demonstrates the importance and feasibility of using a patient-specific viscosity in these simulations. Accurately characterizing cerebrovascular hemodynamics in SCA populations has potential for elucidating the pathophysiology of large-vessel occlusion, aneurysms, and tissue damage in these patients.








Similar content being viewed by others
Abbreviations
- ACA:
-
Anterior cerebral artery
- BAS:
-
Basilar artery
- CFD:
-
Computational fluid dynamics
- CVA:
-
Cerebrovascular accident
- HbSS:
-
Homozygous sickle cell anemia
- Hgb S/A:
-
Hemoglobin S or A
- ICA:
-
Internal carotid artery
- ICAM:
-
Intercellular adhesion molecule
- MCA:
-
Middle cerebral artery
- MR(I):
-
Magnetic resonance (imaging)
- OSI:
-
Oscillatory shear index
- \(\overline{{{\text{OSI}}_{{{\text{P}}/{\text{S}}}} }}\) :
-
Spatially-averaged oscillatory shear index simulated using either patient-specific (P) or standard (S) viscosity
- PC-MRI:
-
Phase contrast-MRI
- PCA:
-
Posterior cerebral artery
- SCA:
-
Sickle cell anemia
- SD:
-
Standard deviation
- TNF-α:
-
Tumor necrosis factor-α
- VCAM:
-
Vascular cell adhesion molecule
- WSS:
-
Wall shear stress
- \({\text{WSS}}_{\text{Max}}\) :
-
Maximum wall shear stress value for steady flow or the maximum time-averaged wall shear stress value for pulsatile simulations
- \(\overline{{{\text{WSS}}_{\text{P/S}} }}\) :
-
Spatially-averaged wall shear stress simulated using either patient-specific (P) or standard (S) viscosity under steady flow conditions
- \(\overline{{{\text{WSS}}_{\text{Mean,P/S}} }}\) :
-
Time- and spatially-averaged wall shear stress using either patient-specific (P) or standard (S) viscosity under pulsatile flow conditions
- WSSMean :
-
Time-averaged value of WSS from pulsatile flow simulations
- \({\text{\% WSS}}_{\text{DIF}}\) :
-
The percent difference of time-averaged WSSMean between simulations using patient-specific viscosity and standard viscosity values
- µ P :
-
Patient-specific viscosity value estimated from clinical hematocrit measurements (kg/m-s)
- µ S :
-
Standard blood viscosity typically used for hemodynamics simulations (0.00368 kg/m-s)
References
Adams, R. J., V. C. Mckie, L. Hsu, B. Files, E. Vichinsky, C. Pegelow, M. Abboud, D. Gallagher, A. Kutlar, F. T. Nichols, D. R. Bonds, D. Brambilla, G. Woods, N. Olivieri, C. Driscoll, S. Miller, W. Wang, A. Hurlett, C. Scher, B. Berman, E. Carl, A. M. Jones, E. S. Roach, E. Wright, R. A. Zimmerman, and M. Waclawiw. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N. Engl. J. Med. 1998. https://doi.org/10.1056/NEJM199807023390102.
Aleluia, M. M., T. C. C. Fonseca, R. Q. Souza, F. I. Neves, C. C. da Guarda, R. P. Santiago, B. L. A. Cunha, C. V. B. Figueiredo, S. S. Santana, S. S. da Paz, J. R. D. Ferreira, B. A. V. Cerqueira, and M. S. Gonçalves. Comparative study of sickle cell anemia and hemoglobin SC disease: clinical characterization, laboratory biomarkers and genetic profiles. BMC Hematol. 17:15, 2017. https://doi.org/10.1186/s12878-017-0087-7.
Alemu, Y., and D. Bluestein. Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artif. Org. 31:677–688, 2007.
Alnæs, M. S., J. Isaksen, K.-A. Mardal, B. Romner, M. K. Morgan, and T. Ingebrigtsen. Computation of hemodynamics in the Circle of Willis. Stroke 38:2500–2505, 2007.
Ando, J., H. Tsuboi, R. Korenaga, Y. Takada, N. Toyama-Sorimachi, M. Miyasaka, and A. Kamiya. Shear stress inhibits adhesion of cultured mouse endothelial cells to lymphocytes by downregulating VCAM-1 expression. Am. J. Physiol. 267:C679–C687, 1994. https://doi.org/10.1152/ajpcell.1994.267.3.c679.
Belhassen, L., G. Pelle, S. Sediame, D. Bachir, C. Carville, C. Bucherer, C. Lacombe, F. Gallacteros, and S. Adnot. Endothelial dysfunction in patients with sickle cell disease is related to selective impairment of shear stress-mediated vasodilation. Blood 97:1584–1589, 2001.
Bharadvaj, B. K., R. F. Mabon, and D. P. Giddens. Steady flow in a model of the human carotid bifurcation. Part I-Flow visualization. J Biomech. 15:349–362, 1982. https://doi.org/10.1016/0021-9290(82)90057-4.
Birkeland, P., K. Gardner, R. Kesse-Adu, J. Davies, J. Lauritsen, F. R. Poulsen, C. M. Tolias, and S. L. Thein. Intracranial aneurysms in sickle-cell disease are associated with the hemoglobin SS genotype but not with moyamoya syndrome. Stroke 47:1710–1713, 2016. https://doi.org/10.1161/STROKEAHA.116.012664.
Bisschops, R. H. C., Y. Van Der Graaf, W. P. T. M. Mali, and J. Van Der Grond. High total cerebral blood flow is associated with a decrease of white matter lesions. J. Neurol. 251:1481–1485, 2004. https://doi.org/10.1007/s00415-004-0569-y.
Biswas, D., D. M. Casey, D. C. Crowder, D. A. Steinman, Y. H. Yun, and F. Loth. Characterization of transition to turbulence for blood in a straight pipe under steady flow conditions. J. Biomech. Eng. 138:2016.
Campbell, I. C., J. Ries, S. S. Dhawan, A. A. Quyyumi, W. R. Taylor, and J. N. Oshinski. Effect of inlet velocity profiles on patient-specific computational fluid dynamics simulations of the carotid bifurcation. J. Biomech. Eng. 134:2012. https://doi.org/10.1115/1.4006681.
Caro, C. G., T. Pedley, R. C. Schroter, and W. A. Seed. The Mechanics of the Circulation. New York: Oxford University Press, 1978.
Cebral, J. R., F. Mut, J. Weir, and C. Putman. Quantitative characterization of the hemodynamic environment in ruptured and unruptured brain aneurysms. Am. J. Neuroradiol. 32:145–151, 2011. https://doi.org/10.3174/ajnr.A2419.
Cebral, J. R., C. M. Putman, M. T. Alley, T. Hope, R. Bammer, and F. Calamante. Hemodynamics in normal cerebral arteries: qualitative comparison of 4D phase-contrast magnetic resonance and image-based computational fluid dynamics. J. Eng. Math. 64:367–378, 2009.
Chappell, D. C., S. E. Varner, R. M. Nerem, R. M. Medford, and R. W. Alexander. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ. Res. 82:532–539, 1998. https://doi.org/10.1161/01.RES.82.5.532.
Chien, S., S. Usami, and J. F. Bertles. Abnormal rheology of oxygenated blood in sickle cell anemia. J. Clin. Invest. 49:623–634, 1970. https://doi.org/10.1172/JCI106273.
Chnafa, C., P. Bouillot, O. Brina, B. M. A. Delattre, M. I. Vargas, K. O. Lovblad, V. M. Pereira, and D. A. Steinman. Vessel calibre and flow splitting relationships at the internal carotid artery terminal bifurcation. Physiol. Meas. 38:2044–2057, 2017.
Cignoni, P., M. Callieri, M. Corsini, M. Dellepiane, F. Ganovelli, and G. Ranzuglia. MeshLab: An open-source mesh processing tool. 6th Eurographics Italian Chapter Conference 2008 - Proceedings. 2008; pp. 129–136.
Dampier, C., B. N. Y. Setty, B. Eggleston, D. Brodecki, P. O’Neal, and M. Stuart. Vaso-occlusion in children with sickle cell disease: clinical characteristics and biologic correlates. J. Pediatr. Hematol. Oncol. 26:785–790, 2004. https://doi.org/10.1016/s0084-3954(07)70057-x.
Das, A., J. P. Wansapura, W. M. Gottliebson, and R. K. Banerjee. Methodology for implementing patient-specific spatial boundary condition during a cardiac cycle from phase-contrast MRI for hemodynamic assessment. Med. Image Anal. 19:121–136, 2015. https://doi.org/10.1016/j.media.2014.09.001.
Detmer, F. J., B. J. Chung, F. Mut, M. Pritz, M. Slawski, F. Hamzei-Sichani, D. Kallmes, C. Putman, C. Jimenez, and J. R. Cebral. Development of a statistical model for discrimination of rupture status in posterior communicating artery aneurysms. Acta Neurochir. (Wien). 160:1643–1652, 2018. https://doi.org/10.1007/s00701-018-3595-8.
Detterich, J., T. Alexy, M. Rabai, R. Wenby, A. Dongelyan, T. Coates, J. Wood, and H. Meiselman. Low-shear red blood cell oxygen transport effectiveness is adversely affected by transfusion and further worsened by deoxygenation in sickle cell disease patients on chronic transfusion therapy. Transfusion 53:297–305, 2013. https://doi.org/10.1111/j.1537-2995.2012.03822.x.
Fields, M. E., K. P. Guilliams, D. K. Ragan, M. M. Binkley, C. Eldeniz, Y. Chen, M. L. Hulbert, R. C. McKinstry, J. S. Shimony, K. D. Vo, A. Doctor, H. An, A. L. Ford, and J. M. Lee. Regional oxygen extraction predicts border zone vulnerability to stroke in sickle cell disease. Neurology 90:e1134–e1142, 2018. https://doi.org/10.1212/WNL.0000000000005194.
Gao, L., Y. Hoi, D. D. Swartz, J. Kolega, A. Siddiqui, and H. Meng. Nascent aneurysm formation at the basilar terminus induced by hemodynamics. Stroke 39:2085–2090, 2008. https://doi.org/10.1161/STROKEAHA.107.509422.
Glagov, S., C. Zarins, D. P. Giddens, and D. N. Ku. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch. Pathol. Lab. Med. 112:1019–1031, 1988.
Guilliams, K. P., M. E. Fields, D. K. Ragan, Y. Chen, C. Eldeniz, M. L. Hulbert, M. M. Binkley, J. N. Rhodes, J. S. Shimony, R. C. McKinstry, K. D. Vo, H. An, J. M. Lee, and A. L. Ford. Large-vessel vasculopathy in children with sickle cell disease: a magnetic resonance imaging study of infarct topography and focal atrophy. Pediatr. Neurol. 69:49–57, 2017. https://doi.org/10.1016/j.pediatrneurol.2016.11.005.
Hassell, K. L. Population estimates of sickle cell disease in the U.S. Am. J. Prev. Med. 38(4):S523–21, 2010.
He, X., and D. N. Ku. Pulsatile flow in the human left coronary artery bifurcation: average conditions. J Biomech Eng. 118:74–82, 1996. https://doi.org/10.1115/1.2795948.
Hutchison, J. S., R. Ichord, A. M. Guerguerian, and G. DeVeber. Cerebrovascular disorders. Semin. Pediatr. Neurol. 11:139–146, 2004. https://doi.org/10.1016/j.spen.2004.04.004.
Jutukonda, M. R., C. A. Lee, N. J. Patel, L. T. Davis, S. J. Waddle, M. C. Gindville, S. Pruthi, A. A. Kassim, M. R. DeBaun, M. J. Donahue, and L. C. Jordan. Differential cerebral hemometabolic responses to blood transfusion in adults and children with sickle cell anemia. J. Magn. Reson. Imaging. 49(2):466–477, 2019.
Kamiya, A., and T. Togawa. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 239:H14–H21, 1980. https://doi.org/10.1152/ajpheart.1980.239.1.h14.
Kassim, A. A., S. Pruthi, M. Day, M. Rodeghier, M. C. Gindville, M. A. Brodsky, M. R. Debaun, and L. C. Jordan. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood 127:2038–2040, 2016. https://doi.org/10.1182/blood-2016-01-694562.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation: positive correlation between plaque location and low and oscillating shear stress. Arteriosclerosis. 5:293–302, 1985.
Mannino, R. G., D. R. Myers, B. Ahn, Y. Wang, M. Rollins, H. Gole, A. S. Lin, R. E. Guldberg, D. P. Giddens, L. H. Timmins, and W. A. Lam. Do-it-yourself in vitro vasculature that recapitulates in vivo geometries for investigating endothelial-blood cell interactions. Sci. Rep. 5:12401, 2015. https://doi.org/10.1038/srep12401.
Marshall, I., P. Papathanasopoulou, and K. Wartolowska. Carotid flow rates and flow division at the bifurcation in healthy volunteers. Physiol. Meas. 25:691–697, 2004.
Milner, J. S., J. A. Moore, B. K. Rutt, and D. A. Steinman. Hemodynamics of human carotid artery bifurcations: computational studies with models reconstructed from magnetic resonance imaging of normal subjects. J. Vasc. Surg. 28(1):P143–156, 1998. https://doi.org/10.1016/S0741-5214(98)70210-1.
Moore, J. E., C. Xu, S. Glagov, C. K. Zarins, and D. N. Ku. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110:225–240, 1994. https://doi.org/10.1016/0021-9150(94)90207-0.
Moser, F. G., S. T. Miller, J. A. Bello, C. H. Pegelow, R. A. Zimmerman, W. C. Wang, K. Ohene-Frempong, A. Schwartz, E. P. Vichinsky, D. Gallagher, and T. R. Kinney. The spectrum of brain MR abnormalities in sickle-cell disease: a report from the cooperative study of sickle cell disease. Am. J. Neuroradiol. 17:965–972, 1996.
Nabavizadeh, S. A., A. Vossough, R. N. Ichord, J. Kwiatkowski, B. A. Pukenas, M. J. Smith, P. B. Storm, E. L. Zager, and R. W. Hurst. Intracranial aneurysms in sickle cell anemia: clinical and imaging findings. J. Neurointerv. Surg. 8:434–440, 2016. https://doi.org/10.1136/neurintsurg-2014-011572.
Needleman, J. P., B. N. Setty, L. Varlotta, C. Dampier, and J. L. Allen. Measurement of hemglobin saturation by oxygen in children and adolescents with sickle cell disease. Pediatr. Polmonol. 28(6):423–428, 1999.
Ohene-Frempong, K., S. J. Weiner, L. A. Sleeper, S. T. Miller, S. Embury, J. W. Moohr, D. L. Wethers, C. H. Pegelow, and F. M. Gill. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 91:288–294, 1998. https://doi.org/10.1182/blood.V91.1.288.
Ohtsuka, A., J. Ando, R. Korenaga, A. Kamiya, N. Toyama-Sorimachi, and M. Miyasaka. The effect of flow on the expression of vascular adhesion molecule-1 by cultured mouse endothelial cells. Biochem. Biophys. Res. Commun. 193(1):303–310, 1993. https://doi.org/10.1006/bbrc.1993.1624.
Pedley, T. The Fluid Mechanics of Large Blood Vessels. Cambridge: Cambridge University Press, 1980.
Prohovnik, I., A. Hurlet-Jensen, R. Adams, D. De Vivo, and S. G. Pavlakis. Hemodynamic etiology of elevated flow velocity and stroke in sickle-cell disease. J. Cereb. Blood Flow Metab. 29:803–810, 2009. https://doi.org/10.1038/jcbfm.2009.6.
Rees, D. C., T. N. Williams, and M. T. Gladwin. Sickle-cell disease. Lancet 376(9757):2018–2031, 2010.
Rivera, C. P., A. Veneziani, R. E. Ware, and M. O. Platt. Original research: sickle cell anemia and pediatric strokes: computational fluid dynamics analysis in the middle cerebral artery. Exp. Biol. Med. 241:755–765, 2016. https://doi.org/10.1177/1535370216636722.
Roach, M. R., S. Scott, and G. G. Ferguson. The hemodynamic importance of the geometry of bifurcations in the circle of Willis (Glass Model Studies). Stroke 3:255–267, 1972.
Rothman, S. M., K. H. Fulling, and J. S. Nelson. Sickle cell anemia and central nervous system infarction: a neuropathological study. Ann. Neurol. 20:684–690, 1986.
Sadasivan, C., D. J. Fiorella, H. H. Woo, and B. B. Lieber. Physical factors effecting cerebral aneurysm pathophysiology. Ann. Biomed. Eng. 41:1347–1365, 2013. https://doi.org/10.1007/s10439-013-0800-z.
Schmalzer, E. A., J. O. Lee, A. K. Brown, and S. Usami. Viscosity of mixtures of sickle and normal red cells at varying hematocrit levels: implications for transfusion. Transfusion 27:228–233, 1987. https://doi.org/10.1046/j.1537-2995.1987.27387235626.x.
Sheriff, J., D. Bluestein, G. Girdhar, and J. Jesty. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann. Biomed. Eng. 38:1442–1450, 2010. https://doi.org/10.1007/s10439-010-9936-2.
Steen, R. G., X. Xiong, J. W. Langston, and K. J. Helton. Brain injury in children with sickle cell disease: prevalence and etiology. Ann. Neurol. 54(5):564–572, 2003.
Steen, R. G., X. Xiong, R. K. Mulhern, J. W. Langston, and W. C. Wang. Subtle brain abnormalities in children with sickle cell disease: relationship to blood hematocrit. Ann. Neurol. 45:279–286, 1999.
Steinberg, M. H. Management of sickle cell disease. N. Engl. J. Med. 340:1021–1030, 1999. https://doi.org/10.1056/NEJM199904013401307.
Steinman, D. A., and V. M. Pereira. How patient specific are patient-specific computational models of cerebral aneurysms? An overview of sources of error and variability. Neuosurg. Focus. 47(1):E14, 2019.
Strouse, J. J., L. C. Jordan, S. Lanzkron, and J. F. Casella. The excess burden of stroke in hospitalized adults with sickle cell disease. Am. J. Hematol. 84(9):548–552, 2009.
Tang, D., C. Yang, S. Kobayashi, and D. N. Ku. Effect of a lipid pool on stress/strain distributions in stenotic arteries: 3-D fluid-structure interactions (FSI) models. J. Biomech. Eng. 126:363–370, 2004.
Taylor, C. A., and D. A. Steinman. Image-based modeling of blood flow and vessel wall dynamics: applications, methods and future directions: Sixth international bio-fluid mechanics symposium and workshop, March 28-30, 2008 Pasadena, California. Ann. Biomed. Eng. 38:1188–1203, 2010. https://doi.org/10.1007/s10439-010-9901-0.
Timmins, L. H., D. S. Molony, P. Eshtehardi, M. C. McDaniel, J. N. Oshinski, D. P. Giddens, and H. Samady. Oscillatory wall shear stress is a dominant flow characteristic affecting lesion progression patterns and plaque vulnerability in patients with coronary artery disease. J. R. Soc. Interface 14:20160972, 2017. https://doi.org/10.1098/rsif.2016.0972.
Tropea, B. I., S. Glagov, and C. K. Zarins. Hemodynamics and atherosclerosis. In: Basic science in vascular disease, edited by A. N. Sidawy, B. E. Sumpio, and R. B. depalma. Armonk: Futura Publishing Company Inc, 1997, pp. 107–126.
Václavů, L., Z. A. V. Baldew, S. Gevers, H. J. M. M. Mutsaerts, K. Fijnvandraat, M. H. Cnossen, C. B. Majoie, J. C. Wood, E. VanBavel, B. J. Biemond, P. van Ooij, and A. J. Nederveen. Intracranial 4D flow magnetic resonance imaging reveals altered haemodynamics in sickle cell disease. Br. J. Haematol. 180:432–442, 2018. https://doi.org/10.1111/bjh.15043.
Valen-Sendstad, K., K.-A. Mardal, M. Mortensen, B. A. P. Reif, and H. P. Langtangen. Direct numerical simulation of transitional flow in a patient-specific intracranial aneurysm. J. Biomech. 44:2826–2832, 2011.
Wagner, M. C., J. R. Eckman, and T. M. Wick. Sickle cell adhesion depends on hemodynamics and endothelial activation. J. Lab. Clin. Med. 144:260–267, 2004. https://doi.org/10.1016/j.lab.2004.08.004.
Wake-Buck, A. K., J. C. Gatenby, and J. C. Gore. Hemodynamic characteristics of the vertebrobasilar system analyzed using MRI-based models. PLoS ONE 7(12):2012. https://doi.org/10.1371/journal.pone.0051346.
Wastnedge, E., D. Waters, S. Patel, K. Morrison, M. Y. Goh, D. Adeloye, and I. Rudan. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J. Glob. Health 8:021103, 2018. https://doi.org/10.7189/jogh.08.021103.
Wick, T. M., and J. R. Eckman. Molecular basis of sickle cell-endothelial cell interactions. Curr. Opin Hematol. 3:118–124, 1996. https://doi.org/10.1097/00062752-199603020-00003.
Yushkevich, P. A., J. Piven, H. C. Hazlett, R. G. Smith, S. Ho, J. C. Gee, and G. Gerig. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 31:1116–1128, 2006. https://doi.org/10.1016/j.neuroimage.2006.01.015.
Zarins, C. K., M. A. Zatina, D. P. Giddens, D. N. Ku, and S. Glagov. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J. Vasc. Surg. 5(3):P413–420, 1987. https://doi.org/10.1016/0741-5214(87)90048-6.
Acknowledgments
This work was supported by grants from the American Heart Association (10POST3450046), the National Institutes of Health (5T32 EB001628-07), and the National Center for Advancing Translational Sciences Vanderbilt CTSA award (UL1 TR002243). We are grateful to the Vanderbilt University Institute of Imaging Science (VUIIS) and the Human Imaging Core for providing imaging research resources used in this study.
Funding
American Heart Association (10POST3450046); National Institutes of Health (5T32 EB001628-07); and the National Center for Advancing Translational Sciences Vanderbilt CTSA award (UL1 TR002243).
Data Availability
Upon request, subject to IRB approval.
Conflict of interest
The authors have no conflicts of interest to declare.
Human Studies
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5)
Informed Consent
Informed consent was obtained from all patients included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keller, S.B., Bumpus, J.M., Gatenby, J.C. et al. Characterizing Intracranial Hemodynamics in Sickle Cell Anemia: Impact of Patient-Specific Viscosity. Cardiovasc Eng Tech 13, 104–119 (2022). https://doi.org/10.1007/s13239-021-00559-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-021-00559-2