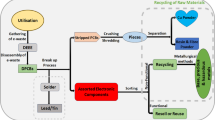
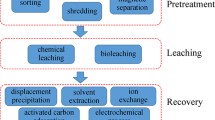
Abstract
The rise in the electronics industry has impacted the environment through the large volumes of waste that are improperly disposed of and the growing demand for precious and rare metals from natural sources. Leaching of copper, cobalt, gold, and silver from printed circuit boards of waste cellular phone has been carried out using imidazolium cation-based ionic liquids (ILs). For the studied metals, the obtaining of selective leaching obtained is reported for the first time, where acidic ionic liquids ([Bmim]HSO4 and [Hmim]HSO4) leached copper and cobalt, while basic ionic liquids ([Bmim]Cl and [Bmim]Br) extracted gold and silver. The effect of temperature has been studied by testing at 60 and 80 °C, where the highest extraction was obtained at the lowest temperature. The concentration of the ionic liquid was also studied through a test without ionic liquid and then varying from 20 to 60%, where at higher concentration the extraction is more efficient ratifying the use of ionic liquids as leaching solutions for metals. Ionic liquids have demonstrated the ability to leach metal ions as the primary reagent in the leaching solution. The following extraction percentages were obtained for each metal: 86.2% copper, 99.5% cobalt, 40.8% gold, and 44.6% silver.



Similar content being viewed by others
References
Zeng X, Yang C, Chiang JF, Li J (2017) Innovating e-waste management: from macroscopic to microscopic scales. Sci Total Environ 575:1–5. https://doi.org/10.1016/j.scitotenv.2016.09.07
Schaeffer N, Passos H, Billard I, Papaiconomou N, Coutinho JAP (2018) Recovery of metals from waste electrical and electronic equipment (WEEE) using unconventional solvents based on ionic liquids. Crit Rev Environ Sci Technol 48(13–15):859–922. https://doi.org/10.1080/10643389.2018.1477417
Yamane LH, de Moraes VT, Espinosa DCR, Tenório JAS (2011) Recycling of WEEE: characterization of spent printed circuit boards from mobile phones and computers. Waste Manag 31(12):2553–2558. https://doi.org/10.1016/J.WASMAN.2011.07.006
Rigoldi A, Trogu EF, Marcheselli GC, Artizzu F, Picone N, Colledani M, Deplano P, Serpe A (2019) Advances in recovering noble metals from waste printed circuit boards (WPCBs). ACS Sustain Chem Eng 7(1):1308–1317. https://doi.org/10.1021/acssuschemeng.8b04983
Tunali M, Tunali MM, Yenigun O (2021) Characterization of different types of electronic waste: heavy metal, precious metal and rare earth element content by comparing different digestıon methods. J Mater Cycles Waste Manag 23(1):149–157. https://doi.org/10.1007/s10163-020-01108-0
Arshadi M, Yaghmaei S, Esmaeili A (2020) Evaluating the optimal digestion method and value distribution of precious metals from different waste printed circuit boards. J Mater Cycles Waste Manag 22(5):1690–1698. https://doi.org/10.1007/s10163-020-01043-0
Jiang P, Song YX, Chen BQ, Harney M, Korzenski MB (2014) Environmentally benign solution for recycling electronic waste using the principles of green chemistry. Adv Mater Res 878:406–412. https://doi.org/10.4028/www.scientific.net/AMR.878.406
Kilicarslan A, Saridede MN, Stopic S, Friedrich B (2014) Use of ionic liquid in leaching process of brass wastes for copper and zinc recovery. Int J Miner Metall Mater 21(2):138–143. https://doi.org/10.1007/s12613-014-0876-y
Huang J, Chen M, Chen H, Chen S, Sun Q (2014) Leaching behavior of copper from waste printed circuit boards with Brønsted acidic ionic liquid. Waste Manag 34(2):483–488. https://doi.org/10.1016/J.WASMAN.2013.10.027
Whitehead JA, Lawrance GA, McCluskey A (2004) “Green” leaching: Recyclable and selective leaching of gold-bearing ore in an ionic liquid. Green Chem. https://doi.org/10.1039/b406148a
Birich A, Mohamed SR, Friedrich B (2018) Screening of non-cyanide leaching reagents for gold recovery from waste electric and electronic equipment. J Sustain Metall 4(2):265–275. https://doi.org/10.1007/s40831-018-0160-x
Marra A, Cesaro A, Belgiorno V (2019) Recovery opportunities of valuable and critical elements from WEEE treatment residues by hydrometallurgical processes. Environ Sci Pollut Res 26(19):19897–19905. https://doi.org/10.1007/s11356-019-05406-5
Islam A, Ahmed T, Awual MR, Rahman A, Sultana M, Aziz AA, Monir MU, Teo SH, Hasan M (2020) Advances in sustainable approaches to recover metals from e-waste—a review. J Clean Prod 244:118815. https://doi.org/10.1016/j.jclepro.2019.118815
Holbrey J, Seddon K (1999) Ionic liquids. Clean Prod Process 1:223–236. https://doi.org/10.1007/s100980050036
Wilkes JS (2004) Properties of ionic liquid solvents for catalysis. J Mol Catal A: Chem 214(1):11–17. https://doi.org/10.1016/J.MOLCATA.2003.11.029
Tshibangu PN, Ndwandwe SN, Dikio ED (2011) Density, viscosity and conductivity study of 1-butyl-3-methylimidazolium bromide. Int J Electrochem Sci 6(6):2201–2213
Grishina EP, Ramenskaya LM, Gruzdev MS, Kraeva OV (2013) Water effect on physicochemical properties of 1-butyl-3-methylimidazolium based ionic liquids with inorganic anions. J Mol Liq 177(3):267–272. https://doi.org/10.1016/j.molliq.2012.10.023
Dharaskar SA, Varma MN, Shende DZ, Yoo CK, Wasewar KL (2013) Synthesis, characterization and application of 1-butyl-3 methylimidazolium chloride as green material for extractive desulfurization of liquid fuel. Sci World J. https://doi.org/10.1155/2013/395274
Alvarez JD, Martínez R, Acosta RB (2012) Líquidos iónicos: propiedades fisicoquímicas y aplicación potencial en el mejoramiento de crudos pesados. Rev ION 25:61
MacFarlane DR, Tachikawa N, Forsyth M, Pringle JM, Howlett PC, Elliott GD, Davis JH, Watanabe M, Simon P, Angell CA (2014) Energy applications of ionic liquids. Energy Environ Sci 7(1):232–250. https://doi.org/10.1039/C3EE42099J
Wishart JF (2009) Energy applications of ionic liquids. Energy Environ Sci 2(9):956–961. https://doi.org/10.1039/b906273d
Dong T, Hua Y, Zhang Q, Zhou D (2009) Leaching of chalcopyrite with Brønsted acidic ionic liquid. Hydrometallurgy 99(1):33–38. https://doi.org/10.1016/j.hydromet.2009.06.001
Carlesi C, Cortes E, Dibernardi G, Morales J, Muñoz E (2016) Ionic liquids as additives for acid leaching of copper from sulfidic ores. Hydrometallurgy 161:29–33. https://doi.org/10.1016/j.hydromet.2016.01.012
Chen M, Wang J, Huang J, Chen H (2015) Behavior of zinc during the process of leaching copper from WPCBs by typical acidic ionic liquids. RSC Adv 5(44):34921–34926. https://doi.org/10.1039/C5RA02655E
He J, Yang J, Tariq SM, Duan C, Zhao Y (2020) Comparative investigation on copper leaching efficiency from waste mobile phones using various types of ionic liquids. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.120368
Zhang DJ, Dong L, Li YT, Wu Y, Ma YX, Yang B (2018) Copper leaching from waste printed circuit boards using typical acidic ionic liquids recovery of e-wastes’ surplus value. Waste Manag 78:191–197. https://doi.org/10.1016/j.wasman.2018.05.036
Matsumoto M, Yamaguchi T, Tahara Y (2020) Extraction of rare earth metal ions with an undiluted hydrophobic pseudoprotic ionic liquid. Metals 10(4):2–7. https://doi.org/10.3390/met10040502
Gras M, Papaiconomou N, Schaeffer N, Chainet E, Tedjar F, Coutinho JAP, Billard I (2018) Ionic-liquid-based acidic aqueous biphasic systems for simultaneous leaching and extraction of metallic ions. Angew Chem Int Ed 57(6):1563–1566. https://doi.org/10.1002/anie.201711068
Billard I (2019) Green solvents in urban mining. Curr Opin Green Sustain Chem 18:37–41. https://doi.org/10.1016/j.cogsc.2018.11.013
Sethurajan M, van Hullebusch ED, Fontana D, Akcil A, Deveci H, Batinic B, Leal JP, Gasche TA, Ali Kucuker M, Kuchta K, Neto IFF, Soares HMVM, Chmielarz A (2019) Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—a review. Crit Rev Environ Sci Technol 49(3):212–275. https://doi.org/10.1080/10643389.2018.1540760
Chen M, Huang J, Ogunseitan OA, Zhu N, Wang Y (2015) Comparative study on copper leaching from waste printed circuit boards by typical ionic liquid acids. Waste Manag 41:142–147. https://doi.org/10.1016/J.WASMAN.2015.03.037
Masilela MD (2018) Application of ionic liquids in the leaching and extraction of precious metals from electronic waste (postgraduate thesis). University of the Witwatersrand, Johannesburg
Namboodiri VV, Varma RS (2002) Solvent-free sonochemical preparation of ionic liquids. Org Lett 4(18):3161–3163. https://doi.org/10.1021/ol026608p
Pereiro AB, Verdía P, Tojo E, Rodríguez A (2007) Physical properties of 1-butyl-3-methylimidazolium methyl sulfate as a function of temperature. J Chem Eng Data 52(2):377–380. https://doi.org/10.1021/je060313v
Tian GC, Feng HK (2014) Study on the physicochemical properties of the mixture of water and 1-butyl-3-methylimidazolium hydrogen sulfate salt ionic liquids. Adv Mater Res 887–888:643–646. https://doi.org/10.4028/www.scientific.net/AMR.887-888.643
Gubbins KE, Walker RD (1965) The solubility and diffusivity of oxygen in electrolytic solutions. J Electrochem Soc 112(5):469. https://doi.org/10.1149/1.2423575
Wang Y, Xu X, Yan Y, Han J, Zhang Z (2010) Phase behavior for the [Bmim]BF4 aqueous two-phase systems containing ammonium sulfate/sodium carbonate salts at different temperatures: Experimental and correlation. Thermochim Acta 501(1–2):112–118. https://doi.org/10.1016/j.tca.2010.01.020
Tanimura K, Amau M, Kume R, Suga K, Okamoto Y, Umakoshi H (2019) Characterization of ionic liquid aqueous two-phase systems: phase separation behaviors and the hydrophobicity index between the two phases. J Phys Chem B 123(27):5866–5874. https://doi.org/10.1021/acs.jpcb.9b04848
Whitehead JA, Zhang J, Pereira N, McCluskey A, Lawrance GA (2007) Application of 1-alkyl-3-methyl-imidazolium ionic liquids in the oxidative leaching of sulphidic copper, gold and silver ores. Hydrometallurgy 88(1–4):109–120. https://doi.org/10.1016/j.hydromet.2007.03.009
Whitehead JA, Zhang J, McCluskey A, Lawrance GA (2009) Comparative leaching of a sulfidic gold ore in ionic liquid and aqueous acid with thiourea and halides using Fe(III) or HSO5- oxidant. Hydrometallurgy 98(3–4):276–280
Zafarani-Moattar M, Hamzehzadeh S (2010) Salting-out effect, preferential exclusion, and phase separation in aqueous solutions of chaotropic water-miscible ionic liquids and kosmotropic salts: effects of temperature, anions, and cations. J Chem Eng Data 55(4):1598–1610. https://doi.org/10.1021/je900681b
Tokuda H, Hayamizu K, Ishii K, Susan MABH, Watanabe M (2004) Physicochemical properties and structures of room temperature ionic liquids. 1. Variation of anionic species. J Phys Chem B 108(42):16593–16600. https://doi.org/10.1021/jp047480r
Tokuda H, Hayamizu K, Ishii K, Susan MABH, Watanabe M (2005) Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J Phys Chem B 109(13):6103–6110. https://doi.org/10.1021/jp044626d
Li F, Chen M (2017) Copper recovery from Waste printed circuit boards and the correlation of Cu, Pb, Zn by ionic liquid. Environ Prot Eng 43(4):55–66. https://doi.org/10.5277/epe170405
Acknowledgements
The authors would like to thank the company Antofagasta SCM for the donation of the printed circuit boards, the Atacama Desert pilot plant center for carrying out the reduction of the solid material, the mining engineering department of the University of Antofagasta, especially Professor Julia Vega, to Liey-Si Wong for the assistance provided for the realization of petrographic microscopy and the organic chemistry laboratory of the University of Antofagasta, particularly Dr. Jorge Borquez. Y. Barrueto thanks the Pontifical Catholic University of Valparaiso for the collaborative work and stay, professors Carlos Carlesi, Pedro Robles, and Álvaro Aracena for their advice. This work was funded by the National Agency for Research and Development (ANID)/National doctorate scholarship and Comisión Nacional de Investigación Científica y Tecnológica (DOCTORADO BECAS CHILE/2018-21180020) and Doctorado en Ingeniería en procesos de minerales de la Universidad de Antofagasta.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barrueto, Y., Hernández, P., Jiménez, Y. et al. Leaching of metals from printed circuit boards using ionic liquids. J Mater Cycles Waste Manag 23, 2028–2036 (2021). https://doi.org/10.1007/s10163-021-01275-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-021-01275-8