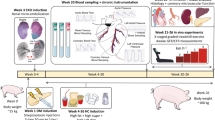
Abstract
Intracellular free Ca2+ ([Ca2+]i) dysregulation occurs in coronary smooth muscle (CSM) in atherosclerotic coronary artery disease (CAD) of metabolic syndrome (MetS) swine. Our goal was to determine how CAD severity, arterial structure, and MetS risk factors associate with [Ca2+]i dysregulation in human CAD compared to changes in Ossabaw miniature swine. CSM cells were dispersed from coronary arteries of explanted hearts from transplant recipients and from lean and MetS swine with CAD. CSM [Ca2+]i elicited by Ca2+ influx and sarcoplasmic reticulum (SR) Ca2+ release and sequestration was measured with fura-2. Increased [Ca2+]i signaling was associated with advanced age and a greater media area in human CAD. Decreased [Ca2+]i signaling was associated with a greater number of risk factors and a higher plaque burden in human and swine CAD. Similar [Ca2+]i dysregulation exhibited in human and Ossabaw swine CSM provides strong evidence for the translational relevance of this large animal model.





Similar content being viewed by others
Abbreviations
- BMI:
-
Body mass index
- [Ca2+]i :
-
Intracellular free calcium
- CSM:
-
Coronary smooth muscle
- CAD:
-
Coronary artery disease
- LDL:
-
Low-density lipoprotein
- LVAD:
-
Left ventricular assist device
- MetS:
-
Metabolic syndrome
- SERCA:
-
Sarco-endoplasmic reticulum Ca2+ ATPase
- SR:
-
Sarcoplasmic reticulum
References
Benjamin, E. J., Virani, S. S., Callaway, C. W., Chamberlain, A. M., Chang, A. R., Cheng, S., et al. (2018). Heart Disease and stroke statistics-2018 update: A report from the American Heart Association. Circulation, 137(12), e67–e492. https://doi.org/10.1161/CIR.0000000000000558.
Wexler, R. K., Elton, T., Pleister, A., & Feldman, D. (2009). Cardiomyopathy: An overview. American Family Physician, 79(9), 778–784.
Libby, P., Ridker, P. M., & Hansson, G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature, 473(7347), 317–325. https://doi.org/10.1038/nature10146.
Owens, G. K., Kumar, M. S., & Wamhoff, B. R. (2004). Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews, 84(3), 767–801. https://doi.org/10.1152/physrev.00041.2003.
van der Schaaf, R. J., Timmer, J. R., Ottervanger, J. P., Hoorntje, J. C., de Boer, M. J., Suryapranata, H., et al. (2006). Long-term impact of multivessel disease on cause-specific mortality after ST elevation myocardial infarction treated with reperfusion therapy. Heart, 92(12), 1760–1763. https://doi.org/10.1136/hrt.2005.086058.
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2016). Heart Disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation, 133(4), e38–e60. https://doi.org/10.1161/CIR.0000000000000350.
Karwowski, W., Naumnik, B., Szczepanski, M., & Mysliwiec, M. (2012). The mechanism of vascular calcification - A systematic review. Medical Science Monitor, 18(1), RA1–RA11.
Hill-Eubanks, D. C., Werner, M. E., Heppner, T. J., & Nelson, M. T. (2011). Calcium signaling in smooth muscle. Cold Spring Harbor Perspectives in Biology, 3(9), a004549. https://doi.org/10.1101/cshperspect.a004549.
Wamhoff, B. R., Bowles, D. K., McDonald, O. G., Sinha, S., Somlyo, A. P., Somlyo, A. V., et al. (2004). L-type voltage-gated Ca2+ channels modulate expression of smooth muscle differentiation marker genes via a rho kinase/myocardin/SRF-dependent mechanism. Circulation Research, 95(4), 406–414. https://doi.org/10.1161/01.RES.0000138582.36921.9e.
Lundberg, M. S., Curto, K. A., Bilato, C., Monticone, R. E., & Crow, M. T. (1998). Regulation of vascular smooth muscle migration by mitogen-activated protein kinase and calcium/calmodulin-dependent protein kinase II signaling pathways. Journal of Molecular and Cellular Cardiology, 30(11), 2377–2389. https://doi.org/10.1006/jmcc.1998.0795.
Pauly, R. R., Bilato, C., Sollott, S. J., Monticone, R., Kelly, P. T., Lakatta, E. G., et al. (1995). Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation, 91(4), 1107–1115. https://doi.org/10.1161/01.cir.91.4.1107.
House, S. J., Potier, M., Bisaillon, J., Singer, H. A., & Trebak, M. (2008). The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflügers Archiv, 456(5), 769–785. https://doi.org/10.1007/s00424-008-0491-8.
Kruse, H. J., Bauriedel, G., Heimerl, J., Hofling, B., & Weber, P. C. (1994). Role of L-type calcium channels on stimulated calcium influx and on proliferative activity of human coronary smooth muscle cells. Journal of Cardiovascular Pharmacology, 24(2), 328–335.
Nilsson, J., Sjolund, M., Palmberg, L., Von Euler, A. M., Jonzon, B., & Thyberg, J. (1985). The calcium antagonist nifedipine inhibits arterial smooth muscle cell proliferation. Atherosclerosis, 58(1-3), 109–122. https://doi.org/10.1016/0021-9150(85)90059-0.
Jiang, H., & Stephens, N. L. (1994). Calcium and smooth muscle contraction. Molecular and Cellular Biochemistry, 135(1), 1–9. https://doi.org/10.1007/BF00925956.
Sturek, M. (2011). Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. Journal of Applied Physiology, 111(2), 573–586. https://doi.org/10.1152/japplphysiol.00373.2011.
Witczak, C. A., Wamhoff, B. R., & Sturek, M. (2006). Exercise training prevents Ca2+ dysregulation in coronary smooth muscle from diabetic dyslipidemic Yucatan swine. Journal of Applied Physiology, 101(3), 752–762. https://doi.org/10.1152/japplphysiol.00235.2006.
Berwick, Z. C., Dick, G. M., O’Leary, H. A., Bender, S. B., Goodwill, A. G., Moberly, S. P., et al. (2013). Contribution of electromechanical coupling between Kv and Ca v1.2 channels to coronary dysfunction in obesity. Basic Research in Cardiology, 108(5), 370. https://doi.org/10.1007/s00395-013-0370-0.
Edwards, J. M., Neeb, Z. P., Alloosh, M. A., Long, X., Bratz, I. N., Peller, C. R., et al. (2010). Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovascular Research, 85(3), 631–640. https://doi.org/10.1093/cvr/cvp308.
Hill, B. J., Price, E. M., Dixon, J. L., & Sturek, M. (2003). Increased calcium buffering in coronary smooth muscle cells from diabetic dyslipidemic pigs. Atherosclerosis, 167(1), 15–23. https://doi.org/10.1016/s0021-9150(02)00381-7.
Neeb, Z. P., Edwards, J. M., Alloosh, M., Long, X., Mokelke, E. A., & Sturek, M. (2010). Metabolic syndrome and coronary artery disease in Ossabaw compared with Yucatan swine. Comparative Medicine, 60(4), 300–315.
McKenney-Drake, M. L., Rodenbeck, S. D., Owen, M. K., Schultz, K. A., Alloosh, M., Tune, J. D., et al. (2016). Biphasic alterations in coronary smooth muscle Ca2+ regulation in a repeat cross-sectional study of coronary artery disease severity in metabolic syndrome. Atherosclerosis, 249, 1–9. https://doi.org/10.1016/j.atherosclerosis.2016.03.032.
Sturek, M., Alloosh, M., & Sellke, F. W. (2020). Swine disease models for optimal vascular engineering. Annual Review of Biomedical Engineering, 22, 25–49. https://doi.org/10.1146/annurev-bioeng-082919-053009.
Badin, J. K., Bruning, R. S., & Sturek, M. (2018). Effect of metabolic syndrome and aging on Ca2+ dysfunction in coronary smooth muscle and coronary artery disease severity in Ossabaw miniature swine. Experimental Gerontology, 108, 247–255. https://doi.org/10.1016/j.exger.2018.04.024.
Badin, J. K., Kole, A., Stivers, B., Progar, V., Pareddy, A., Alloosh, M., et al. (2018). Alloxan-induced diabetes exacerbates coronary atherosclerosis and calcification in Ossabaw miniature swine with metabolic syndrome. Journal of Translational Medicine, 16(1), 58. https://doi.org/10.1186/s12967-018-1431-9.
Institute for Laboratory Animal Research. (2010). Guide for the care and use of laboratory animals. National Academy Press.
AVMA Panel on Euthanasia.American Veterinary Medical Association. (2001). 2000 report of the AVMA panel on euthanasia. JAVMA, 218, 669–696.
Grynkiewicz, G., Poenie, M., & Tsien, R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. J.Biol.Chem., 260, 3440–3450.
Oliver, A. E., Baker, G. A., Fugate, R. D., Tablin, F., & Crowe, J. H. (2000). Effects of temperature on calcium-sensitive fluorescent probes. Biophysical Journal, 78(4), 2116–2126. https://doi.org/10.1016/S0006-3495(00)76758-0.
Dineen, S. L., McKenney, M. L., Bell, L. N., Fullenkamp, A. M., Schultz, K. A., Alloosh, M., et al. (2015). Metabolic syndrome abolishes glucagon-like peptide 1 receptor agonist stimulation of SERCA in coronary smooth muscle. Diabetes, 64(9), 3321–3327. https://doi.org/10.2337/db14-1790.
McKenney-Drake, M. L., Territo, P. R., Salavati, A., Houshmand, S., Persohn, S., Liang, Y., et al. (2016). 18F-NaF PET imaging of early coronary artery calcification. JACC: Cardiovascular Imaging, 9, 627–628. https://doi.org/10.1016/j.jcmg.2015.02.026.
Rodenbeck, S. D., Zarse, C. A., McKenney-Drake, M. L., Bruning, R. S., Sturek, M., Chen, N. X., et al. (2017). Intracellular calcium increases in vascular smooth muscle cells with progression of chronic kidney disease in a rat model. Nephrology, Dialysis, Transplantation, 32(3), 450–458. https://doi.org/10.1093/ndt/gfw274.
Bobe, R., Hadri, L., Lopez, J. J., Sassi, Y., Atassi, F., Karakikes, I., et al. (2011). SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. Journal of Molecular and Cellular Cardiology, 50(4), 621–633. https://doi.org/10.1016/j.yjmcc.2010.12.016.
Karagiannis, G. S., Weile, J., Bader, G. D., & Minta, J. (2013). Integrative pathway dissection of molecular mechanisms of moxLDL-induced vascular smooth muscle phenotype transformation. BMC Cardiovascular Disorders, 13, 4. https://doi.org/10.1186/1471-2261-13-4.
Spillmann, F., Miteva, K., Pieske, B., Tschope, C., & Van Linthout, S. (2015). High-density lipoproteins reduce endothelial-to-mesenchymal transition. Arteriosclerosis, Thrombosis, and Vascular Biology, 35(8), 1774–1777. https://doi.org/10.1161/ATVBAHA.115.305887.
Wang, Y., Ji, L., Jiang, R., Zheng, L., & Liu, D. (2014). Oxidized high-density lipoprotein induces the proliferation and migration of vascular smooth muscle cells by promoting the production of ROS. Journal of Atherosclerosis and Thrombosis, 21(3), 204–216. https://doi.org/10.5551/jat.19448.
Gheorghiade, M., & Bonow, R. O. (1998). Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation, 97(3), 282–289. https://doi.org/10.1161/01.cir.97.3.282.
Bart, B. A., Shaw, L. K., McCants Jr., C. B., Fortin, D. F., Lee, K. L., Califf, R. M., et al. (1997). Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. Journal of the American College of Cardiology, 30(4), 1002–1008. https://doi.org/10.1016/s0735-1097(97)00235-0.
Kim, J. Y., Mun, H. S., Lee, B. K., Yoon, S. B., Choi, E. Y., Min, P. K., et al. (2010). Impact of metabolic syndrome and its individual components on the presence and severity of angiographic coronary artery disease. Yonsei Medical Journal, 51(5), 676–682. https://doi.org/10.3349/ymj.2010.51.5.676.
Gui, M. H., Ling, Y., Liu, L., Jiang, J. J., Li, X. Y., & Gao, X. (2017). Effect of metabolic syndrome score, metabolic syndrome, and its individual components on the prevalence and severity of angiographic coronary artery disease. Chinese Medical Journal (England), 130(6), 669–677. https://doi.org/10.4103/0366-6999.201611.
Ahmadi, A., Leipsic, J., Feuchtner, G., Gransar, H., Kalra, D., Heo, R., et al. (2015). Is metabolic syndrome predictive of prevalence, extent, and risk of coronary artery disease beyond its components? Results from the multinational coronary CT angiography evaluation for clinical outcome: An international multicenter registry (CONFIRM). PLoS One, 10(3), e0118998. https://doi.org/10.1371/journal.pone.0118998.
Miura, Y., Fukumoto, Y., Shiba, N., Miura, T., Shimada, K., Iwama, Y., et al. (2010). Prevalence and clinical implication of metabolic syndrome in chronic heart failure. Circulation Journal, 74(12), 2612–2621. https://doi.org/10.1253/circj.cj-10-0677.
Klotz, S., Jan Danser, A. H., & Burkhoff, D. (2008). Impact of left ventricular assist device (LVAD) support on the cardiac reverse remodeling process. Progress in Biophysics and Molecular Biology, 97(2-3), 479–496. https://doi.org/10.1016/j.pbiomolbio.2008.02.002.
Ootaki, Y., Kamohara, K., Akiyama, M., Zahr, F., Kopcak Jr., M. W., Dessoffy, R., et al. (2005). Phasic coronary blood flow pattern during a continuous flow left ventricular assist support. European Journal of Cardio-Thoracic Surgery, 28(5), 711–716. https://doi.org/10.1016/j.ejcts.2005.08.008.
Symons, J. D., Deeter, L., Deeter, N., Bonn, T., Cho, J. M., Ferrin, P., et al. (2019). Effect of continuous-flow left ventricular assist device support on coronary artery endothelial function in ischemic and nonischemic cardiomyopathy. Circulation: Heart Failure, 12(8), e006085. https://doi.org/10.1161/CIRCHEARTFAILURE.119.006085.
Ambardekar, A. V., Weiser-Evans, M. C. M., Li, M., Purohit, S. N., Aftab, M., Reece, T. B., et al. (2018). Coronary artery remodeling and fibrosis with continuous-flow left ventricular assist device support. Circulation: Heart Failure, 11(5), e004491. https://doi.org/10.1161/CIRCHEARTFAILURE.117.004491.
Wei, X., Li, T., Hagen, B., Zhang, P., Sanchez, P. G., Williams, K., et al. (2013). Short-term mechanical unloading with left ventricular assist devices after acute myocardial infarction conserves calcium cycling and improves heart function. JACC: Cardiovascular Interventions, 6(4), 406–415. https://doi.org/10.1016/j.jcin.2012.12.122.
Acknowledgements
The authors acknowledge the Indiana University School of Medicine Histology Core and Dr. Keith Condon for processing the histology and use of their equipment. Jill K. Badin’s Ph.D. thesis dated August 2019 contained some of the data in this manuscript and can be found at: https://scholarworks.iupui.edu/bitstream/handle/1805/20549/Badin_iupui_0104D_10379.pdf?isAllowed=y&sequence=1
Funding
This research was funded by the National Institutes of Health HL125385, P30 DK097512, the Joshua Diabetes Research Fund, and the Indiana University School of Medicine Center of Excellence in Cardiovascular Research.
Author information
Authors and Affiliations
Contributions
J.K.B., S.D.R., and M.S. are responsible for conception and design of research; J.K.B., C.E., S.D.R., M.A., Z.A.H., I.W., and J.P.G. performed experiments; J.K.B., C.E., and S.D.R. analyzed data; J.K.B., C.E., and M.S. interpreted results of experiments; J.K.B., C.E., and M.S. prepared figures; J.K.B. and C.E. drafted manuscript; J.K.B., C.E., and M.S. edited and revised manuscript; J.K.B., C.E., S.D.R., M.A., Z.A.H., I.W., J.P.G., and M.S. approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval for Use of Animals
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine.
Ethics Approval for Human Subjects
This article does not contain any studies with human participants performed by any of the authors, as approved by exemption, per the use of discarded human tissue, by the Indiana University Institutional Review Board.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Adrian Chester oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Badin, J.K., Eggenberger, C., Rodenbeck, S.D. et al. Intracellular Ca2+ Dysregulation in Coronary Smooth Muscle Is Similar in Coronary Disease of Humans and Ossabaw Miniature Swine. J. of Cardiovasc. Trans. Res. 15, 167–178 (2022). https://doi.org/10.1007/s12265-021-10153-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10153-5