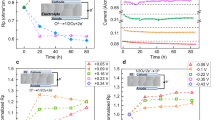
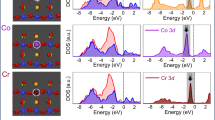
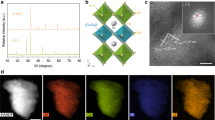
Abstract
Understanding the adsorption and oxidation of NO on metal oxides is of immense interest to environmental and atmospheric (bio)chemistry. Here, we show that the surface oxygen activity, defined as the oxygen 2p-band centre relative to the Fermi level, dictates the adsorption and surface coverage of NOx and the kinetics of NO oxidation for La1−xSrxCoO3 perovskites. Density functional theory and ambient-pressure X-ray photoelectron spectroscopy revealed favourable NO adsorption on surface oxygen sites. Increasing the surface oxygen activity by increasing the strontium substitution led to stronger adsorption and greater storage of NO2, which resulted in more adsorbed nitrogen-like species and molecular nitrogen formed upon exposure to CO. The NO oxidation kinetics exhibited a volcano trend with surface oxygen activity, centred at La0.8Sr0.2CoO3 and with an intrinsic activity comparable to state-of-the-art catalysts. We rationalize the volcano trend by showing that increasing the NO adsorption enhances the oxidation kinetics, although NO adsorption that is too strong poisons the surface oxygen sites with adsorbed NO2 to impede the kinetics.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008).
Delwiche, C. C. The nitrogen cycle. Sci. Am. 223, 136–147 (1970).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167 (2008).
Shiva, S. et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2, 486–493 (2006).
Zhang, X. et al. Managing nitrogen for sustainable development. Nature 528, 51–59 (2015).
Shrimali, M. & Singh, K. P. New methods of nitrate removal from water. Environ. Pollut. 112, 351–359 (2001).
Anenberg, S. C. et al. Impacts and mitigation of excess diesel-related NOx emissions in 11 major vehicle markets. Nature 545, 467–471 (2017).
Takahashi, N. et al. The new concept 3-way catalyst for automotive lean-burn engine: NOx storage and reduction catalyst. Catal. Today 27, 63–69 (1996).
Busca, G., Lietti, L., Ramis, G. & Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review. Appl. Catal. B 18, 1–36 (1998).
Ulissi, Z. W., Medford, A. J., Bligaard, T. & Norskov, J. K. To address surface reaction network complexity using scaling relations machine learning and DFT calculations. Nat. Commun. 8, 14621 (2017).
Medford, A. J. et al. From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J. Catal. 328, 36–42 (2015).
Schlogl, R. Heterogeneous catalysis. Angew. Chem. Int Ed. 54, 3465–3520 (2015).
Friend, C. M. & Xu, B. Heterogeneous catalysis: a central science for a sustainable future. Acc. Chem. Res. 50, 517–521 (2017).
Hematian, S. et al. Nitrogen oxide atom-transfer redox chemistry; mechanism of NO(g) to nitrite conversion utilizing μ-oxo heme-FeIII–O–CuII(L) constructs. J. Am. Chem. Soc. 137, 6602–6615 (2015).
Lee, Y. L., Kleis, J., Rossmeisl, J., Shao-Horn, Y. & Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 4, 3966–3970 (2011).
Jacobs, R., Hwang, J., Shao-Horn, Y. & Morgan, D. Assessing correlations of perovskite catalytic performance with electronic structure descriptors. Chem. Mater. 31, 785–797 (2019).
Grimaud, A. et al. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 4, 2439 (2013).
Hong, W. T. et al. Charge-transfer-energy-dependent oxygen evolution reaction mechanisms for perovskite oxides. Energy Environ. Sci. 10, 2190–2200 (2017).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Viswanathan, B. CO oxidation and NO reduction on perovskite oxides. Catal. Rev. 34, 337–354 (1992).
Chen, J. et al. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B 134–135, 251–257 (2013).
Kim, C. H., Qi, G., Dahlberg, K. & Li, W. Strontium-doped perovskites rival platinum catalysts for treating NOx in simulated diesel exhaust. Science 327, 1624–1627 (2010).
Wang, W. et al. Mixed-phase oxide catalyst based on Mn-mullite (Sm, Gd)Mn2O5 for NO oxidation in diesel exhaust. Science 337, 832–835 (2012).
Hodjati, S., Vaezzadeh, K., Petit, C., Pitchon, V. & Kiennemann, A. Absorption/desorption of NOx process on perovskites: performances to remove NOx from a lean exhaust gas. Appl. Catal. B 26, 5–16 (2000).
Constantinou, C., Li, W., Qi, G. & Epling, W. S. NOX storage and reduction over a perovskite-based lean NOX trap catalyst. Appl. Catal. B 134–135, 66–74 (2013).
Li, X.-G. et al. De-NOx in alternative lean/rich atmospheres on La1−xSrxCoO3 perovskites. Energy Environ. Sci. 4, 3351–3354 (2011).
Chen, J. et al. Catalytic performance of NO oxidation over LaMeO3 (Me = Mn, Fe, Co) perovskite prepared by the sol–gel method. Catal. Commun. 37, 105–108 (2013).
Choi, S. O., Penninger, M., Kim, C. H., Schneider, W. F. & Thompson, L. T. Experimental and computational investigation of effect of Sr on NO oxidation and oxygen exchange for La1−xSrxCoO3 perovskite catalysts. ACS Catal. 3, 2719–2728 (2013).
Penninger, M. W., Kim, C. H., Thompson, L. T. & Schneider, W. F. DFT analysis of NO oxidation intermediates on undoped and doped LaCoO3 perovskite. J. Phys. Chem. C. 119, 20488–20494 (2015).
Zhou, C. et al. NO oxidation catalysis on copper doped hexagonal phase LaCoO3: a combined experimental and theoretical study. Phys. Chem. Chem. Phys. 16, 5106–5112 (2014).
Olsson, L., Persson, H., Fridell, E., Skoglundh, M. & Andersson, B. A kinetic study of NO oxidation and NOx storage on Pt/Al2O3 and Pt/BaO/Al2O3. J. Phys. Chem. B 105, 6895–6906 (2001).
Bhatia, D., McCabe, R. W., Harold, M. P. & Balakotaiah, V. Experimental and kinetic study of NO oxidation on model Pt catalysts. J. Catal. 266, 106–119 (2009).
Wen, Y., Zhang, C., He, H., Yu, Y. & Teraoka, Y. Catalytic oxidation of nitrogen monoxide over La1−xCexCoO3 perovskites. Catal. Today 126, 400–405 (2007).
Suntivich, J. et al. Estimating hybridization of transition metal and oxygen states in perovskites from O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C. 118, 1856–1863 (2014).
Jacobs, R., Mayeshiba, T., Booske, J. & Morgan, D. Material discovery and design principles for stable, high activity perovskite cathodes for solid oxide fuel cells. Adv. Energy Mater. 8, 1702708 (2018).
Grimaud, A. et al. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
Giordano, L. et al. Chemical reactivity descriptor for the oxide-electrolyte interface in Li-ion batteries. J. Phys. Chem. Lett. 8, 3881–3887 (2017).
Onrubia, J. A., Pereda-Ayo, B., De-La-Torre, U. & González-Velasco, J. R. Key factors in Sr-doped LaBO3 (B = Co or Mn) perovskites for NO oxidation in efficient diesel exhaust purification. Appl. Catal. B 213, 198–210 (2017).
Dickens, C. F., Montoya, J. H., Kulkarni, A. R., Bajdich, M. & Nørskov, J. K. An electronic structure descriptor for oxygen reactivity at metal and metal-oxide surfaces. Surf. Sci. 681, 122–129 (2019).
Hammer, B. & Nørskov, J. K. in Impact of Surface Science on Catalysis Vol. 45 (eds Gates, B. C. & Knözinger, H.) 71–129 (Academic, 2000).
Di Valentin, C., Pacchioni, G. & Selloni, A. Electronic structure of defect states in hydroxylated and reduced rutile TiO2(110) surfaces. Phys. Rev. Lett. 97, 166803 (2006).
Giordano, L. et al. Ligand-dependent energetics for dehydrogenation: implications in Li-ion battery electrolyte stability and selective oxidation catalysis of hydrogen-containing molecules. Chem. Mater. 31, 5464–5474 (2019).
Mayeshiba, T. T. & Morgan, D. D. Factors controlling oxygen migration barriers in perovskites. Solid State Ion. 296, 71–77 (2016).
Salmeron, M. & Schlogl, R. Ambient pressure photoelectron spectroscopy: a new tool for surface science and nanotechnology. Surf. Sci. Rep. 63, 169–199 (2008).
Bluhm, H. et al. In situ X-ray photoelectron spectroscopy studies of gas-solid interfaces at near-ambient conditions. MRS Bull. 32, 1022–1030 (2011).
Rosseler, O. et al. Chemistry of NOx on TiO2 surfaces studied by ambient pressure XPS: products, effect of UV irradiation, water, and coadsorbed K+. J. Phys. Chem. Lett. 4, 536–541 (2013).
Rodriguez, J. A. et al. Chemistry of NO2 on oxide surfaces: formation of NO3 on TiO2(110) and NO2↔O vacancy interactions. J. Am. Chem. Soc. 123, 9597–9605 (2001).
Baltrusaitis, J., Jayaweera, P. M. & Grassian, V. H. XPS study of nitrogen dioxide adsorption on metal oxide particle surfaces under different environmental conditions. Phys. Chem. Chem. Phys. 11, 8295–8305 (2009).
Aduru, S., Contarini, S. & Rabalais, J. W. Electron-, x-ray-, and ion-stimulated decomposition of nitrate salts. J. Phys. Chem. 90, 1683–1688 (1986).
Haubrich, J., Quiller, R. G., Benz, L., Liu, Z. & Friend, C. M. In situ ambient pressure studies of the chemistry of NO2 and water on rutile TiO2(110). Langmuir 26, 2445–2451 (2010).
Herranz, T., Deng, X., Cabot, A., Liu, Z. & Salmeron, M. In situ XPS study of the adsorption and reactions of NO and O2 on gold nanoparticles deposited on TiO2 and SiO2. J. Catal. 283, 119–123 (2011).
Au, C. T., Carley, A. F. & Roberts, M. W. Photoelectron spectroscopy: a strategy for the study of reactions at solid surfaces. Int. Rev. Phys. Chem. 5, 57–87 (2008).
Finlayson-Pitts, B. J., Wingen, L. M., Sumner, A. L., Syomin, D. & Ramazan, K. A. The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: an integrated mechanism. Phys. Chem. Chem. Phys. 5, 223–242 (2003).
Morandi, S. et al. Reduction by CO of NOx species stored onto Pt–K/Al2O3 and Pt–Ba/Al2O3 lean NOx traps. Catal. Today 176, 399–403 (2011).
Simonot, L., Garin, F. & Maire, G. A comparative study of LaCoO3, CO3O4 and a mix of LaCoO3—Co3O4: II. Catalytic properties for the CO + NO reaction. Appl. Catal. B 11, 181–191 (1997).
Milt, V. G., Ulla, M. A. & Miró, E. E. NOx trapping and soot combustion on BaCoO3−y perovskite: LRS and FTIR characterization. Appl. Catal. B 57, 13–21 (2005).
Mutoro, E. et al. Reversible compositional control of oxide surfaces by electrochemical potentials. J. Phys. Chem. Lett. 3, 40–44 (2012).
Hwang, J. et al. CO2 reactivity on cobalt-based perovskites. J. Phys. Chem. C. 122, 20391–20401 (2018).
Marques, R. et al. Kinetics and mechanism of steady-state catalytic NO + O2 reactions on Pt/SiO2 and Pt/CeZrO2. J. Mol. Catal. A 221, 127–136 (2004).
Hansen, T. K., Høj, M., Hansen, B. B., Janssens, T. V. W. & Jensen, A. D. The effect of Pt particle size on the oxidation of CO, C3H6, and NO Over Pt/Al2O3 for diesel exhaust aftertreatment. Top. Catal. 60, 1333–1344 (2017).
Dickens, C. F. & Nørskov, J. K. A theoretical investigation into the role of surface defects for oxygen evolution on RuO2. J. Phys. Chem. C. 121, 18516–18524 (2017).
Rao, R. R. et al. Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. 3, 516–525 (2020).
Wu, T. et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2, 763–772 (2019).
Lee, Y.-L. et al. Kinetics of oxygen surface exchange on epitaxial Ruddlesden–Popper phases and correlations to first-principles descriptors. J. Phys. Chem. Lett. 7, 244–249 (2016).
Zaanen, J., Sawatzky, G. A. & Allen, J. W. Band gaps and electronic structure of transition-metal compounds. Phys. Rev. Lett. 55, 418–421 (1985).
Inoue, I. H., Goto, O., Makino, H., Hussey, N. E. & Ishikawa, M. Bandwidth control in a perovskite-type 3d1-correlated metal Ca1−xSrxVO3. Evolution of the electronic properties and effective mass. Phys. Rev. B 58, 4372–4383 (1998).
Hwang, J. et al. Tuning perovskite oxides by strain: electronic structure, properties, and functions in (electro)catalysis and ferroelectricity. Mater. Today 31, 100–118 (2019).
Zaanen, J. & Sawatzky, G. A. Systematics in band gaps and optical spectra of 3D transition metal compounds. J. Solid State Chem. 88, 8–27 (1990).
Crumlin, E. J. et al. Surface strontium enrichment on highly active perovskites for oxygen electrocatalysis in solid oxide fuel cells. Energy Environ. Sci. 5, 6081–6088 (2012).
Feng, Z. et al. Revealing the atomic structure and strontium distribution in nanometer-thick La0.8Sr0.2CoO3−δ grown on (001)-oriented SrTiO3. Energy Environ. Sci. 7, 1166–1174 (2014).
Imamura, M., Matsubayashi, N. & Shimada, H. Catalytically active oxygen species in La1−xSrxCoO3−δ studied by XPS and XAFS spectroscopy. J. Phys. Chem. B 104, 7348–7353 (2000).
González Tejuca, L., Bell, A. T., Fierro, J. L. G. & Peña, M. A. Surface behaviour of reduced LaCoO3 as studied by TPD of CO, CO2 and H2 probes and by XPS. Appl. Surf. Sci. 31, 301–316 (1988).
Tanuma, S., Powell, C. J. & Penn, D. R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 21, 165–176 (1994).
Brown, W. A. & King, D. A. NO chemisorption and reactions on metal surfaces: a new perspective. J. Phys. Chem. B 104, 2578–2595 (2000).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Anisimov, V. I., Aryasetiawan, F. & Lichtenstein, A. I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: the LDA+U method. J. Phys. Condens. Matter 9, 767 (1997).
Lee, Y.-L., Kleis, J., Rossmeisl, J. & Morgan, D. Ab initio energetics of LaBO3 (001) (B = Mn, Fe, Co, and Ni) for solid oxide fuel cell cathode. Phys. Rev. B 80, 224101 (2009).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA+U framework. Phys. Rev. B 73, 195107 (2006).
Stoerzinger, K. A. et al. Reactivity of perovskites with water: role of hydroxylation in wetting and implications for oxygen electrocatalysis. J. Phys. Chem. C. 119, 18504–18512 (2015).
NIST-JANAF Thermochemical Tables (National Institute of Standards and Technology, 1985); https://doi.org/10.18434/T42S31
Towns, J. et al. XSEDE: accelerating scientific discovery. Comput. Sci. Eng. 16, 62–74 (2014).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Acknowledgements
The ALS beamlines 9.3.2 and 11.0.2 are supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences and Materials Sciences Division of the US DOE at the Lawrence Berkeley National Laboratory under Contract DE-AC02-05CH11231. Some of the PLD film growth was conducted at the Center for Nanophase Materials Sciences, a DOE Office of Science User Facility. This research used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the US Department of Energy under Contract No. DE-AC02-05CH11231. This work also used resources of the Extreme Science and Engineering Discovery Environment (XSEDE)84, which is supported by National Science Foundation grant number ACI-1548562. X.R.W. acknowledges support from the Nanyang Assistant Professorship grant from Nanyang Technological University, Academic Research Fund Tier 1 (RG108/17) and (RG177/18) from the Singapore Ministry of Education.
Author information
Authors and Affiliations
Contributions
Y.S.-H., J.H. and R.R.R. conceived and designed the experiments of this projects. J.H., R.R.R., K.A., E.J.C. and H.B. performed the ambient-pressure XPS measurements. X.R.W. grew the epitaxial thin films. K.A. performed the plug-flow reactor measurements. L.G. conducted the density functional theory calculations. Y.S.-H., J.H., R.R.R. and L.G. wrote the manuscript. All authors discussed, commented on and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Catalysis thanks Juan González-Velasco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–41, Tables 1–6 and Notes 1–3.
Supplementary Data 1
Atomic coordinates of optimized structures for DFT calculations conducted in this study.
Rights and permissions
About this article
Cite this article
Hwang, J., Rao, R.R., Giordano, L. et al. Regulating oxygen activity of perovskites to promote NOx oxidation and reduction kinetics. Nat Catal 4, 663–673 (2021). https://doi.org/10.1038/s41929-021-00656-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-021-00656-4
This article is cited by
-
Activating lattice oxygen in NiFe-based (oxy)hydroxide for water electrolysis
Nature Communications (2022)
-
Promoting biomass electrooxidation via modulating proton and oxygen anion deintercalation in hydroxide
Nature Communications (2022)