Abstract
Infection-induced aversion against enteropathogens is a conserved sickness behaviour that can promote host survival1,2. The aetiology of this behaviour remains poorly understood, but studies in Drosophila have linked olfactory and gustatory perception to avoidance behaviours against toxic microorganisms3,4,5. Whether and how enteric infections directly influence sensory perception to induce or modulate such behaviours remains unknown. Here we show that enteropathogen infection in Drosophila can modulate olfaction through metabolic reprogramming of ensheathing glia of the antennal lobe. Infection-induced unpaired cytokine expression in the intestine activates JAK–STAT signalling in ensheathing glia, inducing the expression of glial monocarboxylate transporters and the apolipoprotein glial lazarillo (GLaz), and affecting metabolic coupling of glia and neurons at the antennal lobe. This modulates olfactory discrimination, promotes the avoidance of bacteria-laced food and increases fly survival. Although transient in young flies, gut-induced metabolic reprogramming of ensheathing glia becomes constitutive in old flies owing to age-related intestinal inflammation, which contributes to an age-related decline in olfactory discrimination. Our findings identify adaptive glial metabolic reprogramming by gut-derived cytokines as a mechanism that causes lasting changes in a sensory system in ageing flies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the Article and its Supplementary Information. Raw sequencing reads and pre-processed sequence data for bulk RNA-seq files have been deposited in the Gene Expression Omnibus (GEO) under accession code GSE168530. Raw scRNA-seq reads and preprocessed sequence data have been deposited in the GEO under accession code GSE168572. Source data are provided with this paper.
Code availability
Analysis code for scRNA-seq datasets is available at https://github.com/Hongjie-Li/flyglia. Drosophila genome (version BDGP6) is available for download at https://aug2017.archive.ensembl.org/info/data/ftp/index.html. Drosophila genome (r6.10) is available for download at http://ftp.flybase.net/releases/. The published scRNA-seq datasets generated by the S. Aerts laboratory are available at https://scope.aertslab.org.
References
Wang, A. et al. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 166, 1512–1525 (2016).
Ayres, J. S. & Schneider, D. S. The role of anorexia in resistance and tolerance to infections in Drosophila. PLoS Biol. 7, e1000150 (2009).
Kobler, J. M., Rodriguez Jimenez, F. J., Petcu, I. & Grunwald Kadow, I. C. Immune receptor signaling and the mushroom body mediate post-ingestion pathogen avoidance. Curr. Biol. 30, 4693–4709.e3 (2020).
Stensmyr, M. C. et al. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151, 1345–1357 (2012).
Charroux, B., Daian, F. & Royet, J. Drosophila aversive behavior toward erwinia carotovora carotovora is mediated by bitter neurons and leukokinin. iScience 23, 101152 (2020).
Soria-Gomez, E., Bellocchio, L. & Marsicano, G. New insights on food intake control by olfactory processes: the emerging role of the endocannabinoid system. Mol. Cell. Endocrinol. 397, 59–66 (2014).
Soria-Gómez, E. et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 17, 407–415 (2014).
Liang, L. & Luo, L. The olfactory circuit of the fruit fly Drosophila melanogaster. Sci. China Life Sci. 53, 472–484 (2010).
Liu, H. et al. Astrocyte-like glial cells physiologically regulate olfactory processing through the modification of ORN-PN synaptic strength in Drosophila. Eur. J. Neurosci. 40, 2744–2754 (2014).
Wu, B., Li, J., Chou, Y. H., Luginbuhl, D. & Luo, L. Fibroblast growth factor signaling instructs ensheathing glia wrapping of Drosophila olfactory glomeruli. Proc. Natl Acad. Sci. USA 114, 7505–7512 (2017).
Hussain, A. et al. Inhibition of oxidative stress in cholinergic projection neurons fully rescues aging-associated olfactory circuit degeneration in Drosophila. eLife 7, e32018 (2018).
Wu, S. C., Cao, Z. S., Chang, K. M. & Juang, J. L. Intestinal microbial dysbiosis aggravates the progression of Alzheimer’s disease in Drosophila. Nat. Commun. 8, 24 (2017).
Keshavarzian, A., Engen, P., Bonvegna, S. & Cilia, R. The gut microbiome in Parkinson’s disease: a culprit or a bystander? Prog. Brain Res. 252, 357–450 (2020).
Jiang, H. et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343–1355 (2009).
Tracy Cai, X. et al. AWD regulates timed activation of BMP signaling in intestinal stem cells to maintain tissue homeostasis. Nat. Commun. 10, 2988 (2019).
Bach, E. A. et al. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7, 323–331 (2007).
Freeman, M. R. Drosophila central nervous system glia. Cold Spring Harb. Perspect. Biol. 7, a020552 (2015).
Chakrabarti, S. et al. Remote control of intestinal stem cell activity by haemocytes in Drosophila. PLoS Genet. 12, e1006089 (2016).
Rajan, A. & Perrimon, N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151, 123–137 (2012).
Doty, R. L. & Kamath, V. The influences of age on olfaction: a review. Front. Psychol. 5, 20 (2014).
Li, H., Qi, Y. & Jasper, H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe 19, 240–253 (2016).
Davie, K. et al. A single-cell transcriptome atlas of the aging Drosophila brain. Cell 174, 982–998.e20 (2018).
Liu, L., MacKenzie, K. R., Putluri, N., Maletić-Savatić, M. & Bellen, H. J. The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26, 719–737.e6 (2017).
Fauny, J. D., Silber, J. & Zider, A. Drosophila Lipid Storage Droplet 2 gene (Lsd-2) is expressed and controls lipid storage in wing imaginal discs. Dev. Dyn. 232, 725–732 (2005).
Men, T. T., Binh, T. D., Yamaguchi, M., Huy, N. T. & Kamei, K. Function of Lipid storage droplet 1 (Lsd1) in wing development of Drosophila melanogaster. Int. J. Mol. Sci. 17, E648 (2016).
Kühnlein, R. P. Thematic review series: Lipid droplet synthesis and metabolism: from yeast to man. Lipid droplet-based storage fat metabolism in Drosophila. J. Lipid Res. 53, 1430–1436 (2012).
Soni, K. G. et al. Coatomer-dependent protein delivery to lipid droplets. J. Cell Sci. 122, 1834–1841 (2009).
Ioannou, M. S. et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 177, 1522–1535 (2019).
Liu, L. et al. Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 160, 177–190 (2015).
Rosca, M. G. et al. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 61, 2074–2083 (2012).
Brann, D. H. et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 6, eabc5801 (2020).
Salmon Ceron, D. et al. Self-reported loss of smell without nasal obstruction to identify COVID-19. The multicenter Coranosmia cohort study. J. Infect. 81, 614–620 (2020).
Hakim-Mishnaevski, K., Flint-Brodsly, N., Shklyar, B., Levy-Adam, F. & Kurant, E. Glial phagocytic receptors promote neuronal loss in adult drosophila brain. Cell Rep. 29, 1438–1448.e3 (2019).
Albrecht, S. C., Barata, A. G., Grosshans, J., Teleman, A. A. & Dick, T. P. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819–829 (2011).
Li, H. et al. Classifying drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell 171, 1206–1220.e22 (2017).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Picelli, S. et al. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protocols 9, 171–181 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Anders, S., Pyl, P. T. & Huber, W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 (2015).
Ja, W. W. et al. Prandiology of Drosophila and the CAFE assay. Proc. Natl Acad. Sci. USA 104, 8253–8256 (2007).
Acknowledgements
We thank E. Bach, D. Bilder, S. Hou, M. Zeidler, N. Buchon, D. Ferrandon, L. O’Brien, M. A. Welte, M. Miura, C. S. Thummel and T. Dick for flies; M. Sagolla for the training in Yokogawa CSU-W1/Zeiss 3i Marianas spinning disk confocal microscope; the Genentech NGS technology core lab for the support with bulk RNA-seq; and I. C. Grunwald Kadow for the tips in olfactory T-maze assay. We acknowledge funding from the following grants: National Institutes of Health (NIH) R01AG057353 and Genentech, Inc (H.J.) NIH R00AG062746 (H.L.), NIH K99AG056680 (M.B.J.), NIH R56AG057304 (P.H.), NIH R01DC005982 (L.L.), Howard Hughes Medical Institute (L.L.), C. Zuckerberg Biohub (S.R.Q.) Cancer Prevention & Research Institute of Texas (H.L.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Contributions
X.T.C. and H.J. designed all experiments. H.L. performed scRNA-seq experiments and analysis. L.L. and S.R.Q. provided resources and guidance for scRNA-seq experiments. M.B.J., E.M. and P.H. contributed ideas and experiments to Fig. 1g and Extended Data Fig. 4a–d. J.B. contributed to glia sorting in Extended Data Fig. 7g. Y.L. performed bulk RNA sequencing in Extended Data Fig. 7. X.T.C. performed all other experiments and analysed the data. X.T.C. and H.J. wrote the manuscript with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
X.T.C. is an employee of Genentech Inc., a Roche subsidiary. H.J., J.B. and Y.L. are employees and shareholders of Genentech Inc., a Roche subsidiary. M.B.J. is employee and shareholder of Gordian Biotechnology.
Additional information
Peer review information Nature thanks Matthew Moulton, Scott Pletcher and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Orco and Gr63 odour receptors are required for infection-induced avoidance behaviours towards enteropathogens.
a, Modified CAFE assay used. b, Intake of total food, Ecc15 containing food and normal food for wild-type flies (w1118 × OreR) during homeostasis and 24 h after Ecc15 infection respectively. c, Olfactory T-maze assay and calculation of preference index (P.I.). d, Preference index of young flies 24 h (1d) and 5 days (5d) after Ecc15 infection towards aversive odour, respectively. e, Preference index of wild-type flies (w1118 × OreR) during homeostasis, infected with heat-killed Ecc15 or starved on water for 24 h correspondingly. f, Intake of total food, Ecc15-containing food and normal food for flies in e. g, Intake of total food, Ecc15-containing food and normal food for wild-type flies (w1118 × OreR), Gr63a1 flies, Orco1 flies with or without Ecc15 infection, respectively. h, i, Representative images of 2xSTAT::GFP expression in the central brain 4 h after Ecc15 infection or PE infection, as determined by immunostaining. Anti-GFP antibody amplifies 2xSTAT::GFP signal. Anti-repo immunohistochemistry to label all glia in i. Number quantifications of STAT+ cells per condition are shown in Fig. 1c. Data are mean and s.e.m. The sample size is as follows: n = 7 replicates (3 flies per cohort) per condition in b, n = 5 and 6 independently performed experiments for 1 and 5 days after infection, respectively, in d, n = 7, 8 and 6 independently performed experiments for mock, starved and infected flies with heat-killed Ecc15 in e, n = 10 replicates (3 flies per cohort) per condition in f, n = 7, 10, 8, 9, 10 and 10 replicates (3 flies per cohort) for w1118 mock, w1118 Ecc15, Orco1 mock, Orco1 Ecc15, Gr63a1 mock, Gr63a1 Ecc15 in g. Data shown in f and g are representative of two independently performed experiments, and those shown in b, d, h and i are representative of three separate experiments. P values in b and g from two-tailed Mann–Whitney test; P values in d and f from Dunn’s multiple comparisons test; other P values from Kruskal–Wallis test. NS, not significant (P > 0.9999 in d, f).
Extended Data Fig. 2 Infection does not influence numbers and the morphology of EG at the AL.
a, d, Representative images of 2xSTAT::GFP expression in the EG (nls.mCherry+ driven by GMR56F03::Gal4) in the central brain during homeostasis and 4 h after Ecc15 infection. The AL region was magnified in a and additional images are shown in d. Anti-GFP antibody amplifies the 2xSTAT::GFP signal. Anti-NC82 antibody stained neuropils in d. b, Quantification of 2xSTAT::GFP reporter activity in the EG (nls.mCherry+ in the presence of corresponding Gal4 drivers) during homeostasis and after Ecc15 infection. Numbers of GFP+ mCherry+ cells from both ALs were quantified from 30-μm z-sections (2 μm each). Four different EG-specific Gal4 drivers were tested correspondingly. c, Quantification of EG numbers (nls.mCherry+ driven by SPARC::Gal4, GMR10E12::Gal4 or GMR56F03::Gal4 respectively) at both ALs under mock and infected conditions. e, Histogram overlay of GFP fluorescence in mCherry+ EG in the presence of GMR56F03::Gal4 under conditions as noted, measured by intracellular flow cytometry assay. The GFP fluorescence intensity level (logarithmic scale) is shown in the x axis; the number of events (normalized to its peak height, noted as normalized to modal) is shown on the y axis. Median fluorescence intensity of GFP in mCherry+ EG under these conditions, was computed by FlowJo software and normalized to the median value of mock samples collected on the same day of measurement. mCherry+ EG were sorted by the following gates: (1) forward versus side scatter (FSC versus SSC); (2) side scatter height versus width (SSC-H vs SSC-W); (3) forward scatter height versus width (FSC-H versus FSC-W); (4) fixable viability dye (eFluor 660 to label dead cells before fixation) versus DAPI (labelling nuclei to exclude debris); (5) GFP versus mCherry fluorescence channel (GFP versus mCherry). f, Representative images showing EG morphology at the AL from control and infected flies. EG nuclei were labelled by RedStinger, and cellular processes were labelled by CD4::GFP. Neuropils were labelled by an anti-NC82 antibody . Representative images were generated from 7-μm z-sections (1 μm each) after performing maximal intensity projection. Average intensity levels of CD4::GFP were quantified from 20-μm z-stack confocal images after maximal intensity projection. AL sizes were quantified and normalized to the mean value of mock flies. Data are mean and s.e.m. The sample size is as follows: n = 5, 7, 6, 8, 7, 6, 7 and 7 brains per condition (from left to right) in b, n = 8, 6, 6, 7, 8 and 7 brains per condition (from left to right) in c, n = 97, 135 and 822 mCherry+ cells from mock flies and n = 123, 91, 145 and 163 mCherry+ cells from infected flies in e, n = 9 and 8 brains for mock and Ecc15 flies in f (left), n = 9 brains per condition in f (right). Data in a and d are representative of three independently performed experiments; data shown in b, c, e and f are representative of two independently performed experiments. P values in e from one-tailed Mann–Whitney test; other P values from two-tailed Mann–Whitney test.
Extended Data Fig. 3 JAK–STAT signalling in EG promotes avoidance behaviour against Ecc15, yet increasing host survival upon acute infection.
a, Preference index of young flies with indicated JAK–STAT perturbation in the EG. RNAi constructs were expressed in the EG for 7 days by shifting flies to 29 °C (restrictive temperature for Gal80ts). Flies were exposed to Ecc15 for 24 h. b, Preference index of young infected flies expressing mCherryRNAi, domeRNAi or statRNAi in all glia (repo::Gal4;tubG80ts), measured by T-maze assay. c, Quantification of STAT::GFP activity in the glia of flies after domeRNAi or statRNAi knockdown in all glia (repo::Gal4; 10xSTAT::GFP), to confirm knockdown efficiency for various RNAi lines that target Dome or STAT, respectively. d, e, Total food intake and normal food intake of flies overexpressing hoptuml (d) and of infected flies after knockdown of dome and stat (e) in EG (driven by GMR56F03::Gal4;tubG80ts), measured by CAFE assay. f, Intake of total food, Ecc15-containing food and normal food for flies expressing mCherryRNAi, LacZRNAi and UAS::LacZ in the EG during homeostasis. g, h, Survival curve of flies overexpressing hoptuml (g) or knocking down dome (h) in the EG after continuous PE infection. i, Survival curves of wild-type flies (w1118 × OreR), Gr63a1 flies, Orco1 flies after continuous PE infection. Data are mean and s.e.m. The sample size is as follows: n = 6, 7, 5, 5 and 5 independently performed experiments per condition (from left to right) in a, n = 4 independently performed experiments per condition in b, n = 7, 8, 8, 6 and 7 brains per condition (from left to right) in c, n = 8 and 9 replicates (3 flies per cohort) for mCherryRNAi and hoptuml, respectively in d, n = 6, 8 and 7 replicates (3 flies per cohort) for mCherryRNAi, domeRNAi and statRNAi in e, n = 8 replicates per condition (3 flies per cohort) for f, n = 74 and 96 flies for mCherryRNAi and hoptuml, respectively, for g, n = 97 and 118 flies for mCherryRNAi and domeRNAi, respectively, for h, n = 101, 63 and 90 flies for wild-type flies (w1118 × OreR), Orco1, Gr63a1 flies for i. Data in d, e, g, h are representative of three independently performed experiments; data shown in c, f and i are representative of two independently performed experiments. P values from two-tailed Mann–Whitney test in a (mCherryRNAi compared with hoptuml), c, d; P values from log-rank test in g–i; other P values from Kruskal–Wallis test.
Extended Data Fig. 4 Gut-derived Upd2 and Upd3 are sufficient and required for infection-induced STAT activation in the glia.
a, Gut-derived Upd proteins and their possible effect on the AL. b, Glial expression of nuclear mCherry driven by Mex1::Gal4 in the gut and brain of adult flies. c, d, Activity of 2xSTAT::GFP reporter in the central brain of flies overexpressing upd2, upd3 in enterocytes, driven by Mex1::Gal4;tubG80ts, during homeostasis (c) and of infected flies loss of upd2, upd3 in enterocytes (d). Representative images were generated from 30-μm z-sections, and the AL region was magnified. Numbers of GFP+, repo+ glia per AL were quantified in Fig. 1g. e, f, Preference index of flies expressing mCherryRNAi, UAS::LacZ, LacZRNAi, UAS::upd2, UAS::upd3 and RNAi lines targeting upd2 or upd3 in enterocytes with or without Ecc15 infection. g, qPCR analysis confirming the knockdown efficiency of multiple RNAi lines targeting upd2 or upd3 correspondingly. h, j, Activity of 2xSTAT::GFP reporter in the central brain of flies overexpressing upd2 or upd3 in haemocytes (driven by hm1::Gal4) during homeostasis and of infected flies loss of upd3 in haemocytes. Numbers of GFP+ repo+ cells per AL were quantified from 30-μm z-sections in j, and the AL region was magnified. i, k, Activity of 2xSTAT::GFP reporter in the central brain of flies overexpressing upd2 in fatbody (driven by cg::Gal4) during homeostasis and of infected flies loss of upd3 in fatbody. Numbers of GFP+ repo+ cells per AL were quantified from 30-μm z-sections in k, and the AL region was zoomed in. Data are mean and s.e.m. The sample size is as follows: n = 6, 5, 5, 7, 6 and 9 independently performed experiments per condition (from left to right) in e, n = 4 or 5 independently performed experiments per condition in f, n = 3, 4 and 4 biological replicates per condition (from left to right) in g (left), n = 3, 4 and 2 biological replicates per condition (from left to right) in g (right), n = 12, 14, 11, 9 and 11 brains per condition (from left to right) in j, n = 7, 6, 10 and 13 brains per condition (from left to right) in k. Data in c and d are representative of three independently performed experiments; data shown in b, g–k are representative of two independently performed experiments. P values from two-tailed Mann–Whitney test in f, j and k when comparing two groups; other P values from Kruskal–Wallis test.
Extended Data Fig. 5 Chronic activation of JAK–STAT signalling in EG drives the decline of EG numbers at the AL during ageing.
a, Representative images of glomerular compartments at the AL from young and old flies. Confocal images were generated from 20-μm z-sections (1 μm each) after performing maximal intensity projection. Neuropils labelled by anti-NC82 antibody. b, Representative single z-section images showing EG morphology at the AL from young and old flies. EG nuclei were labelled by RedStinger driven by GMR56F03::Gal4, and cellular processes were labelled by CD4::GFP. Average intensity levels of CD4::GFP and AL sizes were quantified from 20-μm z-stack confocal images after maximal intensity projection. AL sizes were quantified and normalized to the mean values of young flies. c, Representative images of 2xSTAT::GFP reporter activity in EG (nls.mCherry+ driven by GMR56F03::Gal4) at the AL from young and old flies. Images were generated from 20-μm z-sections (1 μm each) after performing maximal intensity projection. d, Preference index of young flies expressing mCherryRNAi in EG (GMR56F03::Gal4), old flies expressing mCherryRNAi, domeRNAi or statRNAi in EG or all glia (repo::Gal4), measured by T-maze assay. e, Representative images showing EG morphology at the AL from old flies with or without dome knockdown. EG nuclei were labelled by RedStinger in the presence of GMR56F03::Gal4;tubG80ts, while cellular processes were labelled by CD4::GFP. Average intensity levels of CD4::GFP and numbers of RedStinger+ cells per AL were quantified. AL sizes were quantified and normalized to the mean value of old control flies. Flies were aged at 25 °C for 14 days followed by 29 °C for 14 days to induce domeRNAi expression. f, Representative images showing the activity of 2xSTAT::GFP reporter in the central brain of old flies knocking down upd2 or upd3 in enterocytes, driven by Mex1::Gal4;tubG80ts. Representative images were generated from 30-μm z-sections, and the AL region was magnified. g, Preference index of young flies expressing mCherryRNAi and old flies expressing mCherryRNAi, Upd2RNAi or Upd3RNAi in enterocytes, driven by Mex1::Gal4;tubG80ts. Data are mean and s.e.m. The sample size is as follows: n = 8 and 6 brains for young and old conditions, respectively, in b, n = 6, 7, 8 and 7 independently performed experiments for young mCherryRNAi, old mCherryRNAi, domeRNAi and statRNAi correspondingly in d (left), n = 4, 3 and 3 independently performed experiments for old mCherryRNAi, domeRNAi and statRNAi correspondingly in d (middle and right), n = 7 and 10 brains for mCherryRNAi, domeRNAi, respectively, in e, n = 5, 7, 7 and 7 independently performed experiments for young mCherryRNAi, old mCherryRNAi, Upd2RNAi and Upd3RNAi, respectively, in g. Data in a–c, f are representative of two independently performed experiments; data in e are representative of three independently performed experiments. P values from two-tailed Mann–Whitney test in b, d (when comparing young and old mCherryRNAi), e, g (when comparing young and old mCherryRNAi); P values from Dunn’s multiple comparisons test in d (right); other P values from Kruskal–Wallis test.
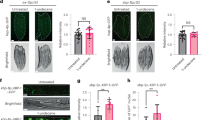
Extended Data Fig. 6 Age-related decline of olfaction sensitivity and morphological decays of EG are independent from microbiota.
a, Preference index of germ-free wild-type flies (w1118 × OreR) during ageing, measured by T-maze assay. b, Representative images of 2xSTAT::GFP reporter activity in the EG at the AL from conventionally-reared or germ-free old flies. EG nuclei were labelled by nls.mCherry in the presence of GMR56F03::Gal4 driver. Anti-GFP antibody amplified 2xSTAT::GFP signal. Anti-NC82 antibody labelled neuropils. Confocal images were generated from 20-μm z-sections after performing maximal intensity projection. Flies were aged at room temperature. c, d, Representative images showing EG morphology at the AL from young and old flies that were conventionally reared and from old germ-free flies, respectively. EG nuclei were labelled by RedStinger in the presence of GMR56F03::Gal4, and cellular processes were labelled by CD4::GFP. Neuropils were labelled by anti-NC82 antibody . Images were generated from 20-μm z-sections after performing maximal intensity projection. Average intensity levels of CD4::GFP and numbers of RedStinger+ cells per AL were quantified in d. AL sizes were quantified and normalized to the mean value of young conventionally reared animals in d. Flies were aged at room temperature. Data are mean and s.e.m. The sample size is as follows: n = 6, 4, 4, 7 and 2 independently performed experiments per condition (from left to right) in a, n = 7 and 8 brains for 30-day conventional and 30-ayd sterile conditions, respectively, in b, n = 6, 8 and 5 brains per condition (from left to right) in d (left), n = 6, 7 and 5 brains per condition (from left to right) in d (middle and right). Data in b–d are representative of two independently performed experiments. P values from Kruskal–Wallis test in a; other P values from two-tailed Mann–Whitney test.
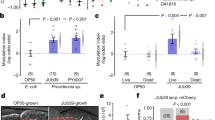
Extended Data Figure 7 JAK–STAT signalling regulates glial lipid metabolism.
a, Workflow of scRNA-seq using plate-based Smart-seq2. FACS, fluorescence-activated cell sorting. Four groups of glia were sequenced: 5 and 50 day all glia (GFP+, driven by rep::Gal4); 5 and 50 day EG (GFP+, driven by GMR56F03::Gal4). b, Visualization of glial cells using t-SNE plots. Cells were coloured according to cell types, ages and Louvain clusters with default resolution. Non-EG were curated from all repo+ glia with EG (GMR56F03::Gal4+) removed (Methods). EG and non-EG were readily separated into different clusters (left and middle). In total, 10 clusters were formed from these glia (right), suggesting the heterogeneity of glial population. c, Violin plot showing expression levels of dome in non-EG and EG. For both EG and non-EG, cells were combined from young and old flies. In non-EG, dome expression was barely detected except in one cell. In EG, a subset of cells showed high expression of dome. d, Violin plots showing expression levels of Socs36E in young and old non-EG (left) and EG (right) respectively. e, Visualization of all annotated glial cells from a previously published whole fly brain scRNA-seq dataset22 using a t-SNE plot. scRNA-seq was performed using droplet-based 10x Genomics platform. Glia are in red (repo+), and neurons are in grey. Two subsets of EG (in orange box) and six subsets of non-EG (in blue box) are annotated. f, Violin plots showing expression levels of Socs36E in non-EG and EG at eight different ages. Cells from 3-, 6- and 9-day-old flies were combined as young samples, and compared with cells from 50-day-old flies (old). g, Gating strategy for sorting STAT::GFP+ glia and STAT::GFP− glia from the central brain of young mock or young infected (4-h Ecc15 infection) flies overexpressing tdTomato in all glia (repo::Gal4) while expressing 10xSTAT::GFP reporter. h, Visualization of gene expression variation between STAT::GFP+ glia and STAT::GFP− glia by PCA plot. Each dot represents a sample replicate independently collected from a cohort of 100 flies. Samples with the same genotype were grouped together, and samples with different treatments were coloured separately. i, Volcano plot displaying differentially expressed genes between STAT::GFP+ glia and STAT::GFP- glia (highlighted in red) under homeostatic conditions, using a cut-off of twofold change, P < 0.001, FDR < 0.01. j, Gene Ontology analysis of significantly upregulated genes in STAT::GFP+ glia during homeostasis. k, Lipid storage-associated genes were significantly upregulated in STAT::GFP+ glia during homeostasis. Reads per kilobase per normalized million mapped reads (nRPKM) values of each gene in STAT::GFP+ glia and STAT::GFP− glia are shown correspondingly. l, Genes involved in monocarboxylate transport were significantly upregulated in STAT::GFP+ glia during homeostasis. nRPKM values of each gene in STAT::GFP+ glia and STAT::GFP− glia are shown correspondingly. m, Schematic demonstrating mitochondrial fatty acid β-oxidation. n, Genes involved in fatty acid β-oxidation that were significantly upregulated in STAT::GFP+ glia during homeostasis. nRPKM values of each gene in STAT::GFP+ glia and STAT::GFP− glia are shown correspondingly. Data are mean and s.e.m. The sample size is as follows: n = 4 replicates per condition (each replicate was independently pooled from 100 flies on different days) in g–n. P values in k, l and n were calculated by Partek Flow; P values in c, d and f from one-tailed Student’s t-test.
Extended Data Fig. 8 JAK–STAT signalling regulates LD accumulation via GLaz and out after infection, with no influence on lipid peroxidation.
a, Immunostaining detecting LDs at the AL from young flies during homeostasis, 24 h after Ecc15 infection, or 4 days after infection, using LipidTox deep red probes. LD numbers per AL were quantified. b, c, Immunostaining detecting LDs at the AL from young flies overexpressing mCherryRNAi or hoptuml in EG (driven by GMR56F03::Gal4;tubG80ts) during homeostasis (b), and from infected flies knocking down dome, stat, GLaz or out in EG (c). LD numbers per AL were quantified. d, Immunostaining detecting LDs at the AL from young flies overexpressing Upd cytokines in enterocytes, driven by Mex1::Gal4;tubG80ts, during homeostasis, and from infected flies knocking down Upd cytokines in enterocytes. LD numbers per AL were quantified. e, f, qPCR analysis confirming the knockdown efficiency of several RNAi lines targeting out, GLaz, NLaz and Ldh, respectively. g, h, Representative images showing lipid peroxidation in LDs at the AL from young flies expressing mCherryRNAi or hoptuml in the EG (driven by GMR56F03::Gal4;tubG80ts) during homeostasis (g), from young mock flies, infected flies or 4 days after Ecc15 infection (h), and from infected flies knocking down dome or stat in the EG (h). Lipid peroxidation levels of LDs for each sample were measured as the mean 488/561 nm intensity ratios in LDs. The ratios were normalized to the mean value of corresponding control samples. Data are mean and s.e.m. The sample size is as follows: n = 10, 7 and 7 brains per condition (from left to right) in a, n = 7 and 5 brains for mCherryRNAi and hoptuml, respectively, in b, n = 13, 7, 7, 9 and 7 brains per condition for mCherryRNAi, domeRNAi, statRNAi, GLazRNAi and outRNAi in c, n = 8, 7, 5, 6, 8 and 8 brains per condition (from left to right) in d, n = 4 and 3 biological replicates for mCherryRNAi and outRNAi in e (left), n = 3 biological replicates for mCherryRNAi and LdhRNAi in e (right), n = 4, 3, 4, 4, 4, 4 and 3 biological replicates per condition (from left to right) in f, n = 6 and 5 brains mCherryRNAi and hoptuml in g, n = 10, 7, 7, 7 and 7 brains per condition (from left to right) in h. Images in a–e, h were generated from 20-μm z-sections (1-μm each) after performing maximal intensity projection. Data in a–d, g, h are representative of three independently performed experiments; data shown in e and f are representative of two separate experiments. P values in a, b, e–g from two-tailed Mann–Whitney test; other P values from Kruskal–Wallis test. NS, not significant (P > 0.9999 in g).
Extended Data Fig. 9 Deactivation of JAK–STAT signalling alleviates lipid toxicity during ageing, thus rescuing the age-related decline of ensheathing glia numbers.
a, Immunostaining detecting LDs in EG (RedStinger+, CD4::GFP+) at the AL of young (5–7 day old) and old (51–54 day old) flies, using LipidTox deep red probes. LD numbers per glia were quantified. b, Cytosolic H2O2 levels in EG at the AL from young (7 day old) and old (50 day old) flies, measured as the mean 405/488 nm intensity ratio. The ratios for old flies were normalized to the mean value for young flies. c–e, Levels of cytosolic H2O2 (c), cytosolic glutathione redox potential (d) and mitochondrial H2O2 (e) in all glia (driven by repo::Gal4) from young and old flies, measured as the mean 405/488 nm intensity ratios for corresponding ROS sensors. f, Representative images showing lipid peroxidation in LDs at the AL from young flies expressing mCherryRNAi in the EG (driven by GMR56F03::Gal4;tubG80ts) and from old flies expressing mCherryRNAi or statRNAi in the EG. LD numbers per AL were quantified. Lipid peroxidation levels of LDs for each sample were measured as the mean 488/561 nm intensity ratio. The ratios were normalized to the mean value of young control samples. Flies were aged at room temperature before being transferred to 29 °C for 7 days. g, Representative images showing lipid peroxidation in LDs at the AL from old flies after loss of upd2 or upd3 in enterocytes driven by Mex1::Gal4;tubG80ts. LD numbers per AL were quantified. Lipid peroxidation levels of LDs for each sample were measured as the mean 488/561 nm intensity ratio. The ratios were normalized to the mean value of old control samples. Data are mean and s.e.m. The sample size is as follows: n = 8 and 6 brains for young and old conditions correspondingly in a and b, n = 6 brains per condition in c, n = 6 and 7 brains for young and old conditions correspondingly in d, n = 7, 7 and 6 brains per condition (from left to right) in e, n = 8, 11 and 8 brains per condition (from left to right) correspondingly in f (left), n = 9, 11 and 8 brains per condition (from left to right) correspondingly in f (right), n = 4, 4 and 6 brains per condition (from left to right) in g (left), n = 5, 5 and 6 brains per condition (from left to right) in g (right). Images in a–g were generated from 20-μm z-sections (1-μm each) after performing maximal intensity projection. Data in a–e are representative of two independently performed experiments, and those in f and g are representative of three separate experiments. P values in a–d, f from two-tailed Mann–Whitney test; other P values from Kruskal–Wallis test.
Extended Data Fig. 10 Inhibiting lipid export or lactate intake in projection neurons partially rescues the decline of olfaction sensitivity upon infection and during ageing.
a, Preference index of young infected flies after knockdown of dome, stat, GLaz or out in the EG with additional RNAi lines. b, c, Intake of total food, Ecc15+ food and normal food for mock flies during homeostasis and for infected flies after the loss of dome, stat, GLaz or out, measured by CAFE assay. The GLazRNAi and outRNAi lines in b and c are different. d, Survival curve of young flies after loss of GLaz or out upon continuous PE infection. The GLazRNAi and outRNAi lines are as in b. e, Representative images showing LD accumulation and lipid peroxidation at the AL from old flies after knockdown of GLaz or out in EG driven by GMR56F03::Gal4;tubG80ts. LD numbers per AL were quantified. Lipid peroxidation levels of LDs for each sample were measured as the mean 488/561 nm intensity ratio. The ratios were normalized to the mean value of old control samples. Flies were aged at 25 °C for 14 days followed by 29 °C for 14 days to induce expression of RNAi lines. f, Preference index values of old flies overexpressing Lip-4, or knockdown of NLaz, Ldh or out in projection neurons using the GH146::Gal4 driver. g, Intake of total food, Ecc15+ food and normal food for young infected flies after knockdown of NLaz or out in projection neurons. h, Survival curve of flies after knockdown of NLaz or out in projection neurons upon continuous PE infection. i, Intake of total food and normal food for young infected flies overexpressing Lip-4 in projection neurons. Data are mean and s.e.m. The sample size is as follows: n = 5, 5, 5, 5, 5, 5 and 4 independently performed experiments per condition (from left to right) in a, n = 6, 6, 5 and 7 replicates (3 flies per cohort) for mock mCherryRNAi, Ecc15 mCherryRNAi, Ecc15 GLazRNAi and Ecc15 outRNAi correspondingly in b, n = 8, 8, 8, 7 and 6 replicates (3 flies per cohort) for mCherryRNAi, domeRNAi, statRNAi, GLazRNAi and outRNAi correspondingly in c, n = 100, 59 and 85 flies for mCherryRNAi, GLazRNAi and outRNAi respectively in d, n = 15, 7 and 7 brains per condition (from left to right) in e, n = 13, 8, 7, 8 and 7 independently performed experiments per condition (from left to right) in f, n = 6, 7, 8 and 7 replicates for mCherryRNAi, NLazRNAi, outRNAi(v51157) and outRNAi(BL67858) correspondingly in g, n = 49, 86, 87 and 53 flies for mCherryRNAi, NLazRNAi, outRNAi(v51157) and outRNAi(BL67858), respectively, in h, n = 8 replicates (3 flies per cohort) per condition in i. Data in b–d, g and h are representative of two independently performed experiments, and those shown in e and i are representative of three separate experiments. P values in d and h from log-rank test; P values in a (when comparing mCherryRNAi, LacZRNAi and UAS::LacZ), c (top: when comparing mCherryRNAi, domeRNAi and GLazRNAi; bottom) and g from Kruskal–Wallis test; other P values from two-tailed Mann–Whitney test.
Supplementary information
Supplementary Figure 1
Example of gating strategy for FACs sorting used in Extended Data Fig. 7g.
Supplemental Table 1
Genotypes, genders and ages of flies used in each figure.
Supplemental Table 2
List of differentially expressed genes in STAT::GFP+ glia compared to STAT::GFP- glia under homeostatic conditions, shown in Extended Data Fig.7i.
Source data
Rights and permissions
About this article
Cite this article
Cai, X.T., Li, H., Borch Jensen, M. et al. Gut cytokines modulate olfaction through metabolic reprogramming of glia. Nature 596, 97–102 (2021). https://doi.org/10.1038/s41586-021-03756-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03756-0
This article is cited by
-
A neuron–glia lipid metabolic cycle couples daily sleep to mitochondrial homeostasis
Nature Neuroscience (2024)
-
Olfactory immunology: the missing piece in airway and CNS defence
Nature Reviews Immunology (2023)
-
Intestine-derived α-synuclein initiates and aggravates pathogenesis of Parkinson’s disease in Drosophila
Translational Neurodegeneration (2022)
-
Sensing microbial infections in the Drosophila melanogaster genetic model organism
Immunogenetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.