Abstract
Purposes
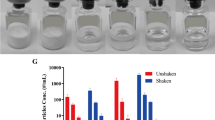
Liquid protein-based biopharmaceutical formulations have been reported to form aggregation and protein sub-visible particles (SbVPs) during dropping (Randolph et al., J Pharm Sci 2015, 104, 602). However, effects of secondary package on liquid biopharmaceutical formulation stability during dropping are overlooked and have not been reported so far. This study reports the first real-world evaluation on effects of secondary package on liquid biopharmaceutical formulation stability during dropping, using two monoclonal antibodies (mAb-1 and mAb-2) and one fusion protein (FP-1) as model biopharmaceuticals.
Methods
The potential protective effects of secondary package and formulation composition on liquid biopharmaceutical formulations during dropping were evaluated with micro-flow imaging (MFI) and dynamic light scattering (DLS).
Results
The dropping-induced degradation could be detected with the two sensitive particle analyzing techniques MFI and DLS. Formulation compositions have dramatic impact on biopharmaceutical stability during dropping. Surprisingly, unlike the primary packages that have been reported to impact liquid biopharmaceutical stability, the secondary packaging system as described in our current preliminary design has little or no protective effect during dropping.
Conclusions
Our study is the first real-world data showing that the secondary package system has little to no effect on the liquid biopharmaceutical formulation quality during dropping. On the contrary, the stability of liquid biopharmaceutical formulations during dropping is more relevant to formulation compositions and primary packages.






Similar content being viewed by others
Abbreviations
- CHO:
-
Chinese Hamster Ovary
- DLS:
-
Dynamic light scattering
- FDA:
-
U.S. Food and Drug Administration
- FP:
-
Fusion protein
- mAb:
-
Monoclonal antibody
- MFI:
-
Micro-flow imaging
- MVSS:
-
MFI View System Suite
- MW:
-
Molecular weight
- PU:
-
Polyurethane
- SbVP:
-
Sub-visible particle
- SP:
-
Secondary package
References
Das TK, Narhi LO, Sreedhara A, Menzen T, Grapentin C, Chou DK, et al. Stress factors in mAb drug substance production processes: critical assessment of impact on product quality and control strategy. J Pharm Sci. 2020;109:116–33.
Nejadnik MR, Randolph TW, Volkin DB, Schöneich C, Carpenter JF, Crommelin DJA, et al. Postproduction handling and administration of protein pharmaceuticals and potential instability issues. J Pharm Sci. 2018;107:2013–9.
Jiskoot W, Nejadnik MR, Sediq AS. Potential issues with the handling of biologicals in a hospital. J Pharm Sci. 2017;106:1688–9.
Ueda T, Nakamura K, Abe Y, Carpenter JF. Effects of product handling parameters on particle levels in a commercial factor VIII product: impacts and mitigation. J Pharm Sci. 2019;108:775–86.
Rosenberg AS. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8:E501–7.
Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;11:99–109.
Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJA, Middaugh CR, Winter G, et al. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci. 2009;98:1201–5.
Singh SK, Afonina N, Awwad M, Bechtold-Peters K, Blue JT, Chou D, et al. An industry perspective on the monitoring of subvisible particles as a quality attribute for protein therapeutics. J Pharm Sci. 2010;99:3302–21.
Bee JS, Goletz TJ, Ragheb JA. The future of protein particle characterization and understanding its potential to diminish the immunogenicity of biopharmaceuticals: a shared perspective. J Pharm Sci. 2012;101:3580–5.
Zölls S, Tantipolphan R, Wiggenhorn M, Winter G, Jiskoot W, Friess W, et al. Particles in therapeutic protein formulations, part 1: overview of analytical methods. J Pharm Sci. 2012;101:914–35.
Docket Number: FDA-1997-D-0145. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/container-closure-systemspackaging-human-drugs-and-biologics
Wang M, Li Y, Srinivasan P, Hu Z, Wang R, Saragih A, et al. Interactions between biological products and product packaging and potential approaches to overcome them. AAPS PharmSciTech. 2018;19:3681–6.
Movafaghi S, Wu H, Francino Urdániz IM, Bull DS, Kelly MD, Randolph TW, et al. The effect of container surface passivation on aggregation of intravenous immunoglobulin induced by mechanical shock. Biotechnol J. 2020;15:e2000096.
Singh R, Lloyd W. A streamlined bioanalytical approach to select a compatible primary container system early in drug development: a toolbox for the biopharmaceutical industry. J Pharm Sci. 2020;109:206–10.
Liu L, Ammar DA, Ross LA, Mandava N, Kahook MY, Carpenter JF. Silicone oil microdroplets and protein aggregates in repackaged bevacizumab and ranibizumab: effects of long-term storage and product mishandling. Invest Ophthalmol Vis Sci. 2011;52:1023–34.
Dill S, Brees K, Stahly A, Cheng E, Carpenter J, Caplan L. Mechanical shock during shipping of medications: the roles of packaging and transportation vendors. J Pharm Sci. 2020;109:670–6.
Randolph TW, Schiltz E, Sederstrom D, Steinmann D, Mozziconacci O, Schöneich C, et al. Do not drop: mechanical shock in vials causes cavitation, protein aggregation, and particle formation. J Pharm Sci. 2015;104:602–11.
Torisu T, Maruno T, Hamaji Y, Ohkubo T, Uchiyama S. Synergistic effect of cavitation and agitation on protein aggregation. J Pharm Sci. 2017;106:521–9.
Duerkop M, Berger E, Dürauer A, Jungbauer A. Impact of cavitation, high shear stress and air/liquid interfaces on protein aggregation. Biotechnol J. 2018;13:e1800062.
Jiao N, Barnett GV, Christian TR, Narhi LO, Joh NH, Joubert MK, et al. Characterization of subvisible particles in biotherapeutic prefilled syringes: the role of polysorbate and protein on the formation of silicone oil and protein subvisible particles after drop shock. J Pharm Sci. 2020;109:640–5.
Wu H, Randolph TW. Aggregation and particle formation during pumping of an antibody formulation are controlled by electrostatic interactions between pump surfaces and protein molecules. J Pharm Sci. 2020;109:1473–82.
Wu H, Chisholm CF, Puryear M, Movafaghi S, Smith SD, Pokhilchuk Y, et al. Container surfaces control initiation of cavitation and resulting particle formation in protein formulations after application of mechanical shock. J Pharm Sci. 2020;109:1270–80.
Valotta Rodrigues R, Puryear M, Sederstrom D, Lengsfeld CS. Parameters influencing cavitation within vials subjected to drop shock. Sci Rep. 2019;9:19210.
Wang H, Zheng H-J, Wang Z, Bai H, Carpenter JF, Chen S, et al. Formation of protein sub-visible particles during vacuum degassing of etanercept solutions. Int J Biol Macromol. 2014;66:151–7.
Fang W-J, Liu J-W, Barnard J, Wang H, Qian Y-C, Xu J. Effects of secondary package on freeze-dried biopharmaceutical formulation stability during dropping. J Pharm Sci. 2021. https://doi.org/10.1016/j.xphs.2021.04.019.
Sharma DK, King D, Oma P, Merchant C. Micro-flow imaging: flow microscopy applied to sub-visible particulate analysis in protein formulations. AAPS J. 2010;12:455–64.
ACKNOWLEDGMENTS AND DISCLOSURES
We greatly appreciate the Ministry of Science and Technology of China (Grant No. 2018ZX09J18107–002) and the National Natural Science Foundation of China (Grant No. 81741144) for financial support. We would also like to extend our gratitude to Ms. Haihong Hu at Zhejiang University for lab management.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, WJ., Liu, JW., Gao, H. et al. Secondary Packages cannot Protect Liquid Biopharmaceutical Formulations from Dropping-Induced Degradation. Pharm Res 38, 1397–1404 (2021). https://doi.org/10.1007/s11095-021-03073-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03073-1