Abstract
The rapid growth in the world population and fast developing industrialization have resulted in the acceleration of environmental pollution due to inadequate treatment methods accompanied by depletion of freshwater. The current research focused on the batch treatment of slaughter house wastewater (SWW) using the sequential three-step electro-coagulation (EC)–electro-oxidation (EO)–adsorption column (AC) processes and to compare the optimized values with the Omani National Standards for the application in irrigation purpose. The characterization of SWW before and after treatment was carried out by measuring chemical oxygen demand (COD), total organic carbon (TOC), total dissolved solids (TDS), turbidity, ammoniacal nitrogen (NH4–N) and conductivity. The optimization of the treatment processes was performed by response surface methodology (RSM) using central composite design. The maximum response obtained using EC unit was 99% with an operating cost of 2.78 USD/m3. The optimum treatment conditions in EC method were found to be 4.0 pH, electrolysis time of 30 min and electrolyte dosage of 5 g/L, with a current density of 18.11 mA/cm2. The maximum reduction in COD was 97% with an operating cost 0.32 USD/m3. The optimum COD reduction in EO method was 84.5% with an operating cost of 6.87 USD/m3. The optimum process parameters in the EO process were observed at 5.0 pH, 56.22 min electrolysis time with 5 g/L electrolyte dosage and a current density of 5 mA/cm2. The response shows 56.27% reduction in COD with an operating cost of 0.088 USD/m3. The study demonstrates that both EC and EO processes for the reduction of COD have a significant effect on the current density. Using adsorption column (AC) studies, the maximum reduction in COD was 76.8% with negligible operating cost. The optimum pH in the case of AC was 7.5, with an effluent flow rate of 8.63 mL/min, and the responses were found to be 76.067%, which indicates both pH and flow rate have significant effect on the % removal of COD.
Similar content being viewed by others
Introduction
Rapid population growth in the sultanate of Oman has resulted in a massive demand for freshwater, which increased to 5.8% from the year 2011 to 2017. Huge production of meat from slaughterhouses generates enormous amounts of wastewater. Nearly 23 m3 of wastewater is discharged in a day from a slaughterhouse located in Suhar and almost 100 m3 of wastewater during the festival season, particularly during Eid season, with a COD value ranges from1500 to 2400 mg/L. Meat processing industries produce huge quantity of slaughter house wastewater after consuming large volume of freshwater during the various processing stages. The discarded wastewater contains considerable amounts of organics and nutrients. As per the regulations from the ministry of environment and climate affairs (MECA) in the sultanate of Oman, the effluent cannot be directly disposed into the public sewage without proper treatment and it is mandatory to bring down the organic level to a permissible limit. Therefore, it is very much essential to treat the slaughterhouse effluent effectively before being discharged into the receiving waters without creating any environmental impact. As per the United States Environmental Protection Agency (US EPA), the SWW plant is considered as one of the major pollution causing industries in the world due to the lack of treatment techniques and hence creates groundwater contamination (United States Environmental Protection Agency, 2017). Thus, a sustainable, cost-effective and environmental friendly treatment method is a prerequisite for the safe disposal of slaughterhouse wastewater to satisfy the MECA regulations. Earlier studies focused on the combined biological and advanced oxidation processes for on-site slaughterhouse wastewater treatment (Barrera et al. 2012; Bustillo‐Lecompte et al. 2016). The organic contaminants present in SWW cause severe environmental pollution and the most widely used treatment methods are anaerobic process, biological and advanced oxidation process. These treatment processes are either too expensive or generate huge quantity of sludge with less efficiency. The incorporation of more advanced and diverse technologies in wastewater treatment applications will result in the generation of less polluted effluent. Such types of water may be recycled for in house utilization during the processing steps. In order to comply with the environmental, health and regulatory frameworks it is required to treat and dispose the effluent in a safe and sustainable release (Bustillo et al. 2015; Bustillo et al. 2013; Bustillo et al. 2014). The characteristics of raw SWW and slaughter house wastewater before treatment are summarized in Table 1.
The disposal of SWW into water bodies influences the water quality with a reduction in dissolved oxygen (DO), which may lead to the depletion of aquatic life (Cao & Mehrvar 2011). In recent times, electro-coagulation process has been receiving considerable attention due to its cost-effectiveness in the removal of metals, suspended solids, oil, grease, organic and inorganic contaminants, etc. present in slaughterhouse effluents. The EC process removes pollutants by inducing an electric current without any chemical addition. Due to this advantage, electro-coagulation is widely recommended as an advanced pre-treatment method for refinery, pharmaceutical and slaughter house wastewater treatment applications (Shaima and Muftah 2014; Victor et al. 2017; Kobya et al. 2006; Bayramoglu et al. 2006). One of the most effective methods to remove organics, nutrients, heavy metals and even pathogens from SWW is electro-coagulation (Qin et al. 2013). EC method was employed to treat chicken processing industry wastewater by introducing an electric current and it was found that the method could effectively remove 92% of COD (Thirugnanasambandham et al. 2014). Combination of electrochemical oxidation, chemical coagulation and biological treatment techniques were applied in the effective removal of contaminants from the highly polluted wastewater (Chakchouk et al. 2017; Mittal 2007). The treatment of textile industry wastewater using three-step process of electro-coagulation, H2O2/Fe2+/UV and adsorption was employed for the effective removal of color with 76% reduction in COD with high yield (Edison GilPavas et al. 2019).
No major studies have been carried out using sequential three-step electro-coagulation, electro-oxidation and adsorption process for the treatment of actual SWW. In this research, a combination of electro-coagulation, electro-oxidation and adsorption process was employed for the effective and environmentally friendly treatment of SWW in Oman. The process conditions are optimized using response surface methodology and results obtained from the optimized values are compared with the Omani National Standard regulation and guidelines for application as irrigation water.
Materials and methods
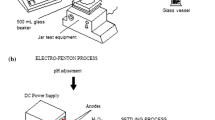
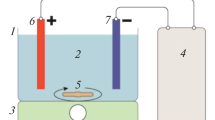
The slaughter house wastewater samples were collected from Suhar Municipal Slaughter house located in North Al Batinah, Oman. Two different types of slaughter house wastewater samples were collected and stored in a refrigerator to avoid degradation. The initial COD of the sample before treatment was 2400 mg/L. Both the EC cell evaluation device and the EO setups were supplied by RT Solutions. The EC cell is constructed of acrylic material with 5.5 cm radius, 18 cm height and a maximum batch capacity of 2000 mL. The electrode assembly consists of aluminum rod (anode) with 1.5 cm radius and 23 cm height. The cathode material used in the EC setup was iron rod with 3.0 cm radius and 25.0 cm height. The electro-coagulation setup is illustrated in Fig. 1 and the EC device is shown in Fig. 2 along with description of various parts of the EC unit. The input AC power supply was 230 V with a maximum operating temperature of 40 °C.
Electro-oxidation setup: The electrode assembly consists of iron and aluminum electrodes coated with titanium (Deepak 2018). The anode has a radius of 1 cm and 14 cm height, while the outer cylindrical hollow cathode is 3 cm radius and 14 cm height. The schematic representation of the EO electrode setup and EO evaluation devices are shown in Figs. 3 and 4.
EO evaluation device (1—electrolyzer (ELECTROGEN), 2—electrolyzer lid with gas vent, 3—electrolyzer tank, 4—transformer–rectifier and control panel, 5—voltmeter, 6—main switch, 7—ammeter, 8—voltage and current adjustment knob, 9—input A/C power cable, 10—DC terminals, 11—output D/C power cable, 12—base frame and 13—electrodes assemble)
Adsorption column setup: Packed bed column was used as the last treating step—polishing step—in order to remove remaining COD. The adsorption column has a dimension of 45 cm × 15 cm and a battery voltage of 12 V. The adsorbent materials used were granular activated carbon, sponge activated carbon and natural zeolite. Natural zeolite was layered to ensure the removal of the last traces of nitrogen. The packed bed adsorption column setup is shown in Fig. 5. Packed bed column was used for the final treatment, acting as a polishing step to reduce the toxicity and remaining organics. A flow meter was inserted in the packed column to adjust the effluent flow rate. The diagrammatic representation of the adsorption column with packing is shown in Fig. 6.
Experimental design and analytical methods
The design of the experimental conditions is performed using Ease STAT expert design to study the response surface methodology and design type is central composite design. Each process was treated in the program separately, though the responses used were COD removal percentage. The percentage reduction in COD is calculated using Eq. (1) (Thirugnanasambandham et al. 2014).
where CODi and CODf are the initial and final COD in mg/L. Other response parameters considered for the study are operating costs for EC and EO. The operating costs in the adsorption column are neglected. The energy consumption and operating cost are calculated using Eqs. (2) and (3), respectively.
where E is the energy consumption in kWh/m3, I is the current in Ampere, V voltage, t is the time of operation in hour and Vsis the volume in liter (Mazoon 2019).
After identification of the process steps and the responses, the independent variables and ranges are selected. The experiments were established based on the central composition composed of 62experiments with six center points each for step 1 and step 2, respectively, and 2 center point for step three. The independent variables of EC and EO are current density; electrolysis time, electrolyte dose and pH have been selected based on the characteristics. Current density was measured using Eq. 4 (Tritt & Schuchardt, 1992).
where I is the electric current in milliampere and Vs is the volume of the solution in cm3.
Results and discussion
Electro-coagulation
Electro-coagulation is the first step of the treatment after the preliminary screening of the SWW, which shows an initial COD of 2400 mg/L. The effluent solution from the EC contains 58 mg/L COD and 204 mg/L TDS, respectively. The color intensity decreased due to the presence of aluminum hydroxides, which acts as a coagulant and forms flocs with hydrocarbons groups in the protein. EC process generates considerable amount of sludge which designates the effectiveness of the treatment process and also the elimination of dark red color. Table 2 represents the process variables and the range of response results. The various electrochemical reactions that take place in the EC process are shown below.
At the anode: \(Al(s) \to Al^{3 + } (aq) + 3e^{ - } E_{0} = + 1.66V\)
At the cathode: \(2H_{2} O(l) + 2e^{ - } \to H_{2} (g) + 2OH^{ - } E_{0} = - 0.83V\).
Effect of solution pH
The initial pH is a major factor influencing the effectiveness of electrochemical process. The pH of the SWW has a direct effect on the solubility of the aluminum hydroxide generated by EC electrode. In the EC experimental study, the initial pH was varied from 4.0 to 6.0 and the percentage reduction in COD was found to be greater at lower pH. The treatment using EC at a pH 4.0 demonstrated excellent pollutant removal efficiency. The pH values giving the maximum percentage removal efficiency are significant in determining the applicability of treatment process. The reason is that the optimum pH can afford the lowest energy consumption which is important in determining the operational cost. At increasing pH, more aluminum dissolution was noticed at the Al–Fe electrode. The result indicates that the EC treatment has been demonstrated to work more efficiently at 4.0 pH. An increase in pH resulted in the participation of the calcium ions in the coagulation of the blood. The results are in agreement with the previous studies (Kobya et al., Kim et al., Syaichurrozi et al. 2021). As the pH increases, the Al–Fe electrode exhibited more aluminum dissolution and this may be due to the active participation of ions in coagulation process and the isoelectric point of blood reached a pH of 6.9. The elevated pH enhances production of more negative charge that may lead to faster denaturation and precipitation process. The electrolysis time and current density have a lesser effect on the solution pH but there is an interaction between pH and electrolyte dosage. The optimum pH for the best electrolysis condition was observed as 4.0 with an optimum dosage of 5 g/L. Figure 7 illustrates the 3D surface plot of the effect of variation of electrolyte dosage and pH on percentage reduction of COD.
Effect of electrolysis time
Electrolysis time is one of the important parameters influencing the reaction rate during the electrochemical treatment process. The EC electrode generates sufficient quantity of dissolved aluminum hydroxide during the initial stages of electrolysis process which indicates the major effect of electrolysis time on COD removal. As a result, the toxic effect will enhance and hence decrease the standard of water quality. This may be due to the formation of oxygen at the anode along with the generation of excess amount of Al(OH)3. Lower toxicity levels were achieved within 5 min of electro-coagulation process and extended treatment time of 30 min or more does not show any toxicity reduction (Kårelid et al. 2017).
Effect of current density
The current density is one of the predominant factors that highly affect the responses with no interaction with other factors. Higher current density allows better separation and reduces the odor and color intensity. On other hand, very high current density allows reduction in passivation of the cathode, with increased energy consumption. The increase in the temperature enhances the voltage and very high current density causes a failure in the electrical conduction. Furthermore, it was observed that the high temperature causes expansion of the parts of the device, leading to water leakage surrounding the electrical connections which results in electric hazards with increased maintenance cost.
Effect of electrolyte dosage
Addition of salt (NaCl) enhances the reaction rate and the SWW displayed low conductivity of 0.88 µS/m2, which is undesirable for an electrochemical system during the ion formation step. The best performance was obtained at an electrolyte dosage corresponding to 5 g/L. It was observed that a good interaction between pH factor and electrolyte dosage factor.
Mathematical model development for % COD removal efficiency (response 1)
Analysis of variance (ANOVA) was employed as a statistical tool for the development of reduced model factors and their interactions. In the response for the percentage COD removal efficiency, the transform power, lambda is 2.3 and the constant is 0. The model F-value of 12.18 implies that the model is significant. There is only a 0.01% chance that an F-value is large due to noise. p values less than 0.0500 indicate that the model terms are significant or experimental parameters show statistical pattern on the COD% response. In this case, B, D, AC, D2 are significant model terms. The lack of fit F-value of 3.62 indicates that the lack of fit is not significant compared to replicate error (pure error). There is a 7.99% chance that a lack of fit F-value is large may be due to the presence of noise. Table 3 indicates the results of ANOVA showing the statistical analysis. There were 30 set of experiments conducted at different settings of combinations of four input factors (pH, electrolysis, dose and density). Residual value of 3.374E + 07 shows that how much variation in COD removal efficiency response is still unexplained. Pure error value 1.106E + 07 in the table indicates the amount of discrepancy between replicate runs.
Final equation of response 1
The equation obtained from central composition experiments was analyzed using multiple regression and quadratic power equation corresponding to 2.3 lambda and 0 constant. An empirical equation was developed to understand the correlation between process independent variables and COD% response. The final models in terms of actual factors are given below:
(COD Removal %)2.3 =—3538.89750 + 4526.69041 × pH – 70.86626 × ET + 8640.3274 × ED + 1928.67296 × CD – 2032.75961 × pH x ED – 33.71682 x (CD)2, where ET = electrolysis time, ED = electrolyte dose and CD = current density.
Optimum conditions
The optimization of processing condition was selected based on the degree of importance of the responses and the goal selected. The common goal was to bring down the values within range. For the response of % COD removal to be maximized, the operating cost to be minimized and the prominent range is 3 out of 5 to all the independent variables, 4 out of 5 for the COD percentage removal and 5 out of 5 for operating cost. The optimum conditions were 4.0 pH, electrolysis time of 30 min, 5 g/L of electrolyte dosage and a current density of 18.11 mA/cm2. The responses were 97% COD removal with an operating cost of 0.84 USD/m3.
Electro-oxidation
Electro-oxidation is the second step of the sequential three-step process. The effluent from EC enters into the EO unit with a COD of 58 mg/L. The EO process demonstrates its ability to lower the COD to a maximum possible extend with a lower operating cost of 0.088 USD/m3. Electro-oxidation process is observed to be slower than EC, which reduces the colloidal and suspended particles by the utilization of less electricity and time (Linares-Hernández et al. 2010). Electro-oxidation process exhibited an excellent performance by breaking the remaining organics. Strong smell of chlorine was sensed after the EO process with an indication of the formation of byproducts during oxidation. Table 4 illustrates the process variables and responses.
Effect of pH
The pH range selected for COD reduction was from 4.0 to 6.0. It was experienced that after treatment using EO has the buffering behavior similar to that of EC process. Also, the buffering behavior attributed to the balance between the consumption and production of hydroxyl ions.
Effect of electrolysis time
The electrolysis time plays an important role on the oxidation of organic contaminants. The oxidation of the pollutants that takes place in the anode shows the best results after 30 min. Electrolysis time has a direct relation with the electrolyte dosage; at low dosages, the percentage COD removal was lower. An increase in electrolysis time will increase the COD removal efficiency. Figure 8 represents the 3D surface plot of the interaction of electrolysis time and electrolyte dose with COD removal.
Effect of current density
Current density is one of the key factors influencing the electro-oxidation process and it has a direct relationship with the percentage COD removal and electrolysis time. The increase in the applied current will enhance the COD reduction at a higher operating cost. The increased current produces more OH radicals by destroying the organic matter present in the pollutants thereby oxidize the surface of the anode.
Effect of electrolyte dose
The increase in the dosage of electrolyte resulted in an increase in the conductivity of the SWW, which shows the efficiency of the process. This is due to the enhanced electrolysis process and improved kinetics or movement of the species and radicals toward the anode. This factor has double interaction with electrolysis time as previously mentioned. The 3D surface plot shown in Fig. 9 represents the interaction of electrolysis time and current density with respect to COD removal.
Development of mathematical model for % COD removal efficiency (response 1)
ANOVA was used to generate the statistical analysis of the reduced model factors and their interactions. The COD removal response has a transform power, lambda 2.17 and constant is 0. The Model f-value of 10.13 implies that Table 5 model is significant. There is only a 0.01% chance that an f-value could occur due to noise. p values less than 0.0500 show the model terms are significant. In this case, B, D, BD, B2 are significant model terms. Table 6 shows the results of ANOVA analysis. There were 30 set of experiments conducted at different settings of combinations of four input factors (pH, electrolysis, dose and density). The lack of fit F-value of 0.4418 indicates that the lack of fit is not significant compared to replicate error (pure error). There is a 90.8% chance that a lack of fit F-value large may be due to the presence of noise. Residual value of 3.939E + 06 shows that how much variation in COD removal efficiency response is still unexplained. Pure error value of 6.996E + 06 in Table 5 indicates the amount of discrepancy between replicate runs (central runs).
Final equation in terms of actual factors
The equation formed from central composition experiments was analyzed using multiple regression and quadratic power equation of 2.17 lambda and 0 constant. One empirical equation was developed to understand the correlation between process independent variables and the response. The variables involved in the equation were electrolyte dose, electrolysis time, current density and the interaction factors which are electrolysis time and current density and electrolysis time as the highest independent factor that affects the process in second order. The final model equation in terms of actual factors is given below.
(COD Removal %)2.17 = -2382.83293 + 153.87854 × ET + 826.73298 × ED + 67.56182 × CD -9.61865 × ET x ED + 1.21127 × ET x CD -0.662501 x (ET) 2, where ET = electrolysis time, ED = electrolyte dose and CD = current density.
Optimization
The optimum conditions were selected based on the degree of importance of the responses and the goal selected. The common goal selected must be in the range and for the response of COD removal % to be maximized and the operating cost to be minimized. The range is 3 out of 5 to all the independent variables are 4 out of 5 for the percentage removal of COD and 5 out of 5 for the operating cost. The optimum conditions are 4.996 pH, electrolysis time of 56.219 min, electrolyte dosage of 5 g/L and current density of 5 mA/cm2. The responses obtained are 56.271% COD removal and an operating cost of 0.088 USD/m3.
Packed bed column
The final stage of the treatment was adsorption process which is considered as the polishing step with granular activated carbon, sponge activated carbon and zeolite as adsorbent for the reduction of nitrogen. Two factors and one response were considered here, which are COD removal efficiency as a response and pH and flow rate as process variables. At this stage, the percentage reduction in COD was from −11% to 76.8%. The operating cost due to adsorption was negligibly small (nearly 0.00026 USD) and hence neglected. The effects of process variables are depicted in Table 6.
Effect of pH
The pH variation of the SWW affects the surface charge of the adsorbent used in the adsorption column and it has a direct impact on the degree of ionization. The pH of the effluent after electro-oxidation process was 6.0 and this pH was not favorable at high effluent flow rate and it increased the COD to 11%. The pH attribute was of curved form in the range of 6.0 to 9.0 and maximum COD removal occurred at a pH of 7.5. The activated carbon was highly pH sensitive. At low pH, mobility of hydrogen ions is higher side as a result; it prevents the adsorption of other metal ions due to the accumulation of H+ at the surface of activated carbon. Figure 10 shows the 3D surface plot of the interaction between pH and flow rate with respect to COD removal.
Effect of flow rate
The flow rate of effluent is a major factor contributes the effectiveness of adsorption in a packed bed column. The flow rates used for the study range 1–10 mL/min. The study shows a direct relation between the flow rate and COD removal. RSM results indicated an interaction between the two factors. However, extra contact time in acidic or basic conditions and high hydrogen ion or hydroxyl ion concentration force to reduce adsorption.
Mathematical model development
The analysis of variance (ANOVA) was used for the statistical analysis of the reduced model factors and their interactions. The COD removal response has the transform power, lambda is 1.13 and constant is 12.1. The model F-value of 275.53 implies the model is significant. There is only a 0.01% chance that an F-value due to noise p values less than 0.0500 indicate model terms are significant. In this case, AB, A2, A2B are significant model terms. Values greater than 0.1000 indicate the model terms are not significant. Table 7 represents the ANOVA analysis. There were 13 set of experiments conducted at different settings of combinations of two input factors (pH and flow rate) with COD removal (%) as the output response (dependent variable). The lack of fit F-value of 0.8727 indicates that the lack of fit is not significant compared to replicate error (pure error). There is a 40.31% chance that a lack of fit F-value is large may be due to the presence of noise. Residual value of 25.40 shows that how much variation in COD removal efficiency response is still unexplained. Pure error values of 26.06 in the table indicate the amount of difference between replicate runs.
Final equation in terms of actual factors
The equation formed from central composition experiments was analyzed using multiple regression and quadratic power equation of 1.13 lambda and 12.1 constant. One empirical equation was developed to recognize the correlation between process independent variables and response, the variables involved in the equation are pH and flow rate, additionally pH was the highest independent factor that affects the process. The final model equations in terms of actual factors are given below:
(COD + 12.10)1.13 = −1579.89653 + 443.29038 × pH −194.75240 × Q + 56.89857 × pH x Q—28.56394 × pH2—1.96043 × Q2—4.04280 × pH2 x Q + 0.226038 × pH x Q2, where Q is the flow rate.
Optimum condition
The optimum condition is selected in design expert software after model has formed based on the degree of importance of the response and the goal selected. The common goal was in the range and for the response COD removal % to be maximized and the importance range is 3 to all the independent variables and 5 for the COD percentage removal. The optimum conditions were obtained at 7.495 pH, 8.63 mL/ min flow rate with a response of 76.067%.
The EC, EO, AC process were operated in sequential mode to accomplish the data by means of RSM and also to generate the model equations. The optimizations of the processing (Tables 8 and 9) conditions were performed through series mode. The color of slaughterhouse effluent before treatment was dark red with an initial COD of 2400 mg/L and ammonium nitrate content was 107 mg/L. The COD of the effluent after third stage treatment process was 6 mg/L corresponding to a pH of 7.0, which shows the effectiveness of the three-stage treatment process. The experimental results of the sequential three-stage process for the reduction COD are shown in Table 10. The maximum percentage reduction in COD was 99.75% using this three-step sequential treatment process with an operating cost of 0.41 USD/m3. Table 8 summarizes the characteristics of the raw effluent and treated effluents in electro-coagulation, electro-oxidation and packed bed adsorption process, respectively.
Omani regulation and standards for irrigation water
Comparing with the Omani standards published for the irrigation as stated in the royal decree no.115/2009, the experimental results using sequential three steps EC–EO–AC are completely satisfied with very good quality. Although the cost of treatment process is very high, it exceeds 0.41 USD/m3 while the selling price of 23 m3 is 0.052USD [Technical Note, 2018].
Table 9 represents the comparison of the parameters after EC–EO–AC process with standard values followed for irrigation water provided by Majis Industrial services in 2019.
Economic evaluation: A simple economic evaluation of EC, EO was carried out by accounting only the operational costs of the energy consumption, chemicals and electrodes costs for the first and second steps only. Tables 10 and 11 illustrate the economic evaluation of the electro-coagulation (EC) and electro-oxidation (EC) processes. The AC step depends on the activated carbon as a whole and the zeolite and its economic evaluation are shown in Table 12.
Overall, the expensive step was EO while the less expensive one was the third step; however, EC has a reasonable operational cost and offers best % COD removal. On the other hand, the costs of electrodes are highly dependent on the material as well as the electricity consumption.
Conclusion
In this research, the sequential electro-coagulation, electro-oxidation and adsorption process was successfully employed for the treatment of slaughterhouse wastewater. The optimizations of the processes were carried out using design expert software for the development of model equations. The Overall reduction in COD using the sequential EC–EO–AC was found to be 99.7% with total estimated operating cost of 0.41 USD/m3. Processed water from the adsorption process could be utilized for irrigation purpose; however, by considering the cost of the proposed treatment, it is suggested to be used on the field implementation such as wastewater treatment plants. The process parameters after the sequential treatment were compared with the Omani regulation and standards for irrigation water and found to be within the limit. The three-step sequential process showed maximum COD removal efficiency, shorter treatment time, low sludge production and reasonable operating cost. The slaughterhouse wastewater treatment using the three-step sequential process was demonstrated to be an efficient and environmentally friendly method.
References
Al-Zdjali S (2016) 10089 Sacrifices in Muscat Municipal Slaughterhouses during Eid.Al Shabiba.17 September [Online] https://www.shabiba.com/article/156840
Barrera M, Mehrvar M, Gilbride K, McCarthy L, Laursen A, Bostan V (2012) Photolytic treatment of organic constituents and bacterial pathogens in secondary effluent of synthetic slaughterhouse wastewater. Chem Eng Res and Design 90(9):1335–1350
Bayramoglu M, Kobya M, Eyvaz M, Senturk E (2006) Technical and economic analysis of electrocoagulation for the treatment of poultry slaughterhouse wastewater. Sep and Purif Tech 51(3):404–408
Bustillo-Lecompte C, Mehrvar M, Quiñones-Bolaños E (2016) Slaughterhouse wastewater characterization and treatment: an economic and public health necessity of the meat processing industry in Ontario. Canada J Geosci and Envir Protec 4:175–186
Bustillo-Lecompte CF, Mehrvar M (2015) Slaughterhouse wastewater characteristics, treatment and management in the meat processing industry: a review on trends and advances. J Env Manag 161:287–302
Bustillo-Lecompte CF, Mehrvar M, Quiñones-Bolaños E (2013) Combined anaerobic- aerobic and UV/H2O2 processes for the treatment of synthetic slaughterhouse waste- water. J Env Scie and Health, Part a: Toxic/hazardous Substances and Envir Eng 48(9):1122–1135
Bustillo-Lecompte CF, Mehrvar M, Quiñones-Bolaños E (2014) Cost-effectiveness analysis of TOC removal from slaughterhouse wastewater using combined anaerobic-aerobic and UV/H2O2 processes. J Env Manag 134:145–152
Cao W & Mehrvar M (2011) Slaughterhouse wastewater treatment by combined anaerobic baffled reactor and UV/H2O2 processes. Chem Eng Res and Design. 89(7):1136–1143
Chakchouk I, Elloumi N, Belaid C, Mseddi S, Chaari L, Kallel M (2017) A combined electrocoagulation–electrooxidation treatment for dairy wastewater. Braz J Chem Eng 34(01):109–117
Deepak S (2018). Electrocoagulation & Electrooxidation evaluation devices. [Interview] 19 September, 2018.
El-Naas M, Abu Alhaij M, Al-Zuhair S (2014) Evaluation of a three-step process for the treatment of petroleum refinery Wastewater. J Env Chem Eng 2:56–62
GilPavas E, Dobrosz-Gómez I, Gómez-García MÁ (2019) Optimization and toxicity assessment of a combined electrocoagulation, H2O2/Fe2+/UV and activated carbon adsorption for textile wastewater treatment. Science of the Total Env 651:551–560
Kårelid V, Larsson G, Berndt Bj€orlenius, (2017) Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. Env Manag 193:491–502
Kim KJ, Baek K, Ji S, Cheong Y, Yim G, Jang A (2016) Study on electrocoagulation parameters (current density, pH and electrode distance) for removal of fluoride from groundwater. Environ Earth Sci 75:45–56
Kobya M, Senturk E, Bayramoglu M (2006) Treatment of poultry slaughterhouse wastewaters by electrocoagulation. J Haz Mat 133(1–3):172–176
Linares-Hernández I, Barrera-Díaz C, Bilyeu B, Juárez-García Rojas P, Campos-Medina E (2010) A combined electrocoagulation–electrooxidation treatment for industrial wastewater. J Haz Mat 175(3):688–694. https://doi.org/10.1016/j.jhazmat.2009.10.064
Mazoon electricity company (2019) Services Prices [online] available from: http://mzec.co.om/Default.aspx
Mittal GS (2007) Treatment of wastewater from abattoirs before land application - A review Biores. Tech 97(9):1119–1135
Qin X, Yang B, Gao F, Chen G (2013) Treatment of restaurant wastewater by pilot-scale electrocoagulation- electro flotation: optimization of operating conditions. J Environ Eng 139(7):1004–1016
Syaichurrozi I, Sarto S, Sediawan WB, Hidayat M (2021) Effect of Current and Initial pH on Electrocoagulation in Treating the Distillery Spent Wash with Very High Pollutant Content. Water 2021, 13(11).https://dx.doi.org/https://doi.org/10.3390/w13010011.
Shaima SH, Muftah HE (2014) Characterization of the removal of Chromium(VI) from groundwater by electrocoagulation. J Indus Eng Chem 20(5):2775–2781. https://doi.org/10.1016/j.jiec.2013.11.006.
Technical note on Water Quality Protection, Environment Regulatory Department, Sultanate of Oman (2018).
Thirugnanasambandham K, Sivakumar V, Prakash Maran J (2014) Response surface modeling and optimization of treatment of meat industry wastewater using electrochemical treatment method. J Taiwan Inst of Chem Eng 2:688–694
Tritt WP, Schuchardt F (1992) Materials flow and possibilities of treating liquid and solid wastes from slaughterhouses in Germany. A Review Biores Tech 41(3):235–245
United States Environmental Protection Agency (US EPA). Effluent limitations guidelines and new source performance standards for the meat and poultry products point source category [Internet]. 2004 [Updated: 2004‐10‐08]. Available from: https:// federalregister.gov/a/04‐12017 [Accessed: 2016‐06‐22]
Victor K, Gen Larsson B, Bjorlenius, (2017) Pilot-scale removal of pharmaceuticals in municipal wastewater: Comparison of granular and powdered activated carbon treatment at three wastewater treatment plants. Env Manag 30:1–12
Acknowledgements
This research was supported by M/s. Majis Industrial Services Company, Oman. The research team expresses their sincere appreciation to M/s. Majis Industrial Services for providing the laboratory facilities and materials to carry out the research.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Saidi, A.S.K., Rashidi, N.N.S.A., Devi, M.G. et al. Sequential three-step process for the treatment of slaughter house wastewater and its optimization using response surface modeling studies. Appl Water Sci 11, 139 (2021). https://doi.org/10.1007/s13201-021-01469-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-021-01469-3