Abstract
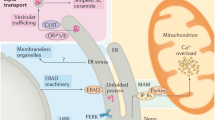
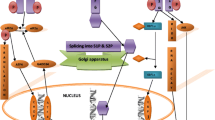
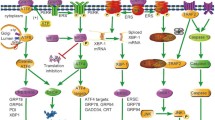
The endoplasmic reticulum (ER) is an organelle that orchestrates the production and proper assembly of an extensive types of secretory and membrane proteins. Endoplasmic reticulum stress is conventionally related to prolonged disruption in the protein folding machinery resulting in the accumulation of unfolded proteins in the ER. This disruption is often manifested due to oxidative stress, Ca2+ leakage, iron imbalance, disease conditions which in turn hampers the cellular homeostasis and induces cellular apoptosis. A mild ER stress is often reverted back to normal. However, cells retaliate to acute ER stress by activating the unfolded protein response (UPR) which comprises three signaling pathways, Activating transcription factor 6 (ATF6), inositol requiring enzyme 1 alpha (IRE1α), and protein kinase RNA-activated-like ER kinase (PERK). The UPR response participates in both protective and pro-apoptotic responses and not much is known about the mechanistic aspects of the switch from pro‐survival to pro‐apoptosis. When ER stress outpaces UPR response then cell apoptosis prevails which often leads to the development of various diseases including cardiomyopathies. Therefore, it is important to identify molecules that modulate the UPR that may serve as promising tools towards effective treatment of cardiovascular diseases. In this review, we elucidated the latest advances in construing the contribution imparted by the three arms of UPR to combat the adverse environment in the ER to restore cellular homeostasis during cardiomyopathies. We also summarized the various therapeutic agents that plays crucial role in tilting the UPR response towards pro-survival.


Similar content being viewed by others
References
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529. https://doi.org/10.1038/nrm2199
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110(10):1389–1398. https://doi.org/10.1172/JCI16886
Pereira BC, da Rocha AL, Pinto AP, Pauli JR, de Souza CT, Cintra DE, Ropelle ER, de Freitas EC, Zagatto AM, da Silva AS (2016) Excessive eccentric exercise-induced overtraining model leads to endoplasmic reticulum stress in mice skeletal muscles. Life Sci 145:144–151. https://doi.org/10.1016/j.lfs.2015.12.037
Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149. https://doi.org/10.1152/physrev.00015.2006
Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL (2015) Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res 59:292–307. https://doi.org/10.1111/jpi.12264
Moran AE, Forouzanfar MH, Roth GA, Mehsah GA, Ezzati M, Murray CJ, Naghavi M (2010) Temporal trends in ischemic heart disease mortality in 21 world regions, 1980 to 2010: the Global Burden of Disease 2010 study. Circulation 129(14):1483–1492. https://doi.org/10.1161/circulationaha.113.004042
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P (2017) Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135(10):e146–e603. https://doi.org/10.1161/cir.0000000000000485
Crossman DC (2004) The pathophysiology of myocardial ischaemia. Heart 90(5):576–580. https://doi.org/10.1136/hrt.2003.029017
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357(11):1121–1135. https://doi.org/10.1056/nejmra071667
Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE (2006) Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291(3):H1411–H1420. https://doi.org/10.1152/ajpheart.01378.2005
Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC (2006) Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 99(3):275–282. https://doi.org/10.1161/01.res.0000233317.70421.03
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev 7:1013–1030. https://doi.org/10.1038/nrd2755
Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27:315–327. https://doi.org/10.1038/sj.emboj.7601974
Pizzo P, Pozzan T (2007) Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 17(10):511–517. https://doi.org/10.1016/j.tcb.2007.07.011
Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS (2003) Global analysis of protein expression in yeast. Nature 425:737–741. https://doi.org/10.1038/nature02046
Gaut JR, Hendershot LM (1993) The modification and assembly of proteins in the endoplasmic reticulum. Curr Opin Cell Biol 5:589–595. https://doi.org/10.1016/0955-0674(93)90127-C
Sanderson TH, Gallaway M, Kumar R (2015) Unfolding the unfolded protein response: unique insights into brain ischemia. Int J Mol Sci 16(4):7133–7142. https://doi.org/10.3390/ijms16047133
Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789. https://doi.org/10.1146/annurev.biochem.73.011303.074134
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2(6):326–332. https://doi.org/10.1038/35014014
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13:365–376. https://doi.org/10.1016/j.devcel.2007.07.018
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10:3787–3799. https://doi.org/10.1091/mbc.10.11.3787
Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21(3):396–413. https://doi.org/10.1089/ars.2014.5851
Chen X, Shen J, Prywes R (2002) The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 277:13045–13052. https://doi.org/10.1074/jbc.M110636200
Nadanaka S, Yoshida H, Mori K (2006) Reduction of disulfide bridges in the lumenal domain of ATF6 in response to glucose starvation. Cell Struct Funct 31(2):127–134. https://doi.org/10.1247/csf.06024
Nadanaka S, Okada T, Yoshida H, Mori K (2007) Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 27(3):1027–1043. https://doi.org/10.1128/mcb.00408-06
Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell 6:1355–1364. https://doi.org/10.1016/S1097-2765(00)00133-7
Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R (2000) Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 275(35):27013–27020. https://doi.org/10.1016/S0021-9258(19)61473-0
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904. https://doi.org/10.1016/S1097-2765(00)80330-5
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176. https://doi.org/10.1016/S1097-2765(01)00265-9
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96. https://doi.org/10.1038/415092a
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881–891. https://doi.org/10.1016/S0092-8674(01)00611-0
Groenendyk J, Agellon LB, Michalak M (2013) Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol 75:49–67. https://doi.org/10.1146/annurev-physiol-030212-183707
Yan B, Liu S, Li X, Zhong Y, Tong F, Yang S (2019) Preconditioning with endoplasmic reticulum stress alleviated heart ischemia/reperfusion injury via modulating IRE1/ATF6/RACK1/PERK and PGC-1α in diabetes mellitus. Biomed Pharmacother 118:109407. https://doi.org/10.1016/j.biopha.2019.109407
Toldo S, Severino A, Abbate A, Baldi A (2011) The role of PDI as a survival factor in cardiomyocyte ischemia. Methods Enzymol 489:47–65. https://doi.org/10.1016/B978-0-12-385116-1.00003-0
Zeng L, Lu M, Mori K, Luo S, Lee AS, Zhu Y, Shyy JY (2004) ATF6 modulates SREBP2-mediated lipogenesis. EMBO J 23:950–958. https://doi.org/10.1038/sj.emboj.7600106
Sawada T, Minamino T, Fu HY, Asai M, Okuda K, Isomura T, Yamazaki S, Asano Y, Okada K, Tsukamoto O, Sanada S, Asanuma H, Asakura M, Takashima S, Kitakaze M, Komuro I (2010) X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J Mol Cell Cardiol 48:1280–1289. https://doi.org/10.1016/j.yjmcc.2010.02.004
Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabò R, Di Lisa F, Gorza L (2003) Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J 17:923–925. https://doi.org/10.1096/fj.02-0644fje
Zhao L, Jiang S, Wu N, Shi E, Yang L, Li Q (2021) MiR-17-5p-mediated endoplasmic reticulum stress promotes acute myocardial ischemia injury through targeting Tsg101. Cell Stress Chaperones 26(1):77–90. https://doi.org/10.1007/s12192-020-01157-2
Gao G, Xie A, Zhang J, Herman AM, Jeong EM, Gu L, Liu M, Yang KC, Kamp TJ, Dudley SC (2013) Unfolded protein response regulates cardiac sodium current in systolic human heart failure. Circ Arrhythm Electrophysiol 6:1018–1024. https://doi.org/10.1161/circep.113.000274
Liu M, Dudley S (2015) Role for the unfolded protein response in heart disease and cardiac arrhythmias. Int J Mol Sci 17:52. https://doi.org/10.3390/ijms17010052
Liu X, Kwak D, Lu Z, Xu X, Fassett J, Wang H, Wei Y, Cavener DR, Hu X, Hall J, Bache RJ, Chen Y (2014) Endoplasmic reticulum stress sensor protein kinase R-like endoplasmic reticulum kinase (PERK) protects against pressure overload-induced heart failure and lung remodeling. Hypertension 64(4):738–744. https://doi.org/10.1161/hypertensionaha.114.03811
Liu Z, Cai H, Zhu H, Toque H, Zhao N, Qiu C, Guan G, Dang Y, Wang J (2014) Protein kinase RNA-like endoplasmic reticulum kinase (PERK)/calcineurin signaling is a novel pathway regulating intracellular calcium accumulation which might be involved in ventricular arrhythmias in diabetic cardiomyopathy. Cell Signal 26(12):2591–2600. https://doi.org/10.1038/cddis.2015.183
Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T (2004) Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol 24:8007–8017. https://doi.org/10.1128/mcb.24.18.8007-8017.2004
Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F (2010) Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS ONE 5:e9575. https://doi.org/10.1371/journal.pone.0009575
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z (2007) Involvement of endoplasmic reticulum stress in myocardial apoptosis of streptozocin-induced diabetic rats. J Clin Biochem Nutr 41(1):58–67. https://doi.org/10.3164/jcbn.2007008
Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L (2009) Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med 13(8A):1499–1512. https://doi.org/10.1111/j.1582-4934.2009.00833.x
Younce CW, Wang K, Kolattukudy PE (2010) Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res 87(4):665–674. https://doi.org/10.1093/cvr/cvq102
Miki T, Miura T, Hotta H, Tanno M, Yano T, Sato T, Terashima Y, Takada A, Ishikawa S, Shimamoto K (2009) Endoplasmic reticulum stress in diabetic hearts abolishes erythropoietin-induced myocardial protection by impairment of phospho-glycogen synthase kinase-3beta-mediated suppression of mitochondrial permeability transition. Diabetes 58(12):2863–2872. https://doi.org/10.2337/db09-0158
Wei H, Zhang R, Jin H, Liu D, Tang X, Tang C, Du J (2010) Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum stress in rats. Antioxid Redox Signal 12(9):1079–1091. https://doi.org/10.1089/ars.2009.2898
Zhou G, Li X, Hein DW, Xiang X, Marshall JP, Prabhu SD, Cai L (2008) Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol 52(8):655–666. https://doi.org/10.1016/j.jacc.2008.05.019
Wu T, Dong Z, Geng J, Sun Y, Liu G, Kang W, Zhang Y, Ge Z (2011) Valsartan protects against ER stress-induced myocardial apoptosis via CHOP/Puma signaling pathway in streptozotocin-induced diabetic rats. Eur J Pharm Sci 42(5):496–502. https://doi.org/10.1016/j.ejps.2011.02.005
Kranstuber AL, Del Rio C, Biesiadecki BJ, Hamlin RL, Ottobre J, Gyorke S, Lacombe VA (2012) Advanced glycation end product cross-link breaker attenuates diabetes-induced cardiac dysfunction by improving sarcoplasmic reticulum calcium handling. Front Physiol 3:292. https://doi.org/10.3389/fphys.2012.00292
Liu Z, Zhu H, Ma Y, Tang Z, Zhao N, Wang Y, Pan S (2021) AGEs exacerbates coronary microvascular dysfunction in NoCAD by activating endoplasmic reticulum stress-mediated PERK signaling pathway. Metabolism 117:154710. https://doi.org/10.1016/j.metabol.2021.154710
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2001) Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol Cell Biol 21(4):1239–1248. https://doi.org/10.1128/mcb.21.4.1239-1248.2001
Thuerauf DJ, Morrison L, Glembotski CC (2004) Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J Biol Chem 279(20):21078–21084. https://doi.org/10.1074/jbc.M400713200
Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC (2007) Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. J Biol Chem 282(31):22865–22878. https://doi.org/10.1074/jbc.M701213200
Pieper LA, Strotbek M, Wenger T, Olayioye MA, Hausser A (2017) ATF6β-based fine-tuning of the unfolded protein response enhances therapeutic antibody productivity of Chinese hamster ovary cells. Biotechnol Bioeng 114(6):1310–1318. https://doi.org/10.1002/bit.26263
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7(9):880–885. https://doi.org/10.1038/sj.embor.7400779
Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC (2006) Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res 98(9):1186–1193. https://doi.org/10.1161/01.res.0000220643.65941.8d
Blackwood EA, Azizi K, Thuerauf DJ, Paxman RJ, Plate L, Kelly JW, Wiseman RL, Glembotski CC (2019) Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. Nat Commun 10(1):187. https://doi.org/10.1038/s41467-018-08129-2
Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC (2009) Ischemia activates the ATF6 branch of the endoplasmic reticulum stress response. J Biol Chem 284(43):29735–29745. https://doi.org/10.1074/jbc.M109.018036
Jin JK, Blackwood EA, Azizi K, Thuerauf DJ, Fahem AG, Hofmann C, Kaufman RJ, Doroudgar S, Glembotski CC (2017) ATF6 decreases myocardial ischemia/reperfusion damage and links ER stress and oxidative stress signaling pathways in the heart. Circ Res 120(5):862–875. https://doi.org/10.1161/circresaha.116.310266
Toko H, Takahashi H, Kayama Y, Okada S, Minamino T, Terasaki F, Kitaura Y, Komuro I (2010) ATF6 is important under both pathological and physiological states in the heart. J Mol Cell Cardiol 49(1):113–120. https://doi.org/10.1016/j.yjmcc.2010.03.020
Thuerauf DJ, Hoover H, Meller J, Hernandez J, Su L, Andrews C, Dillmann WH, McDonough PM, Glembotski CC (2001) Sarco/endoplasmic reticulum calcium ATPase-2 expression is regulated by ATF6 during the endoplasmic reticulum stress response: intracellular signaling of calcium stress in a cardiac myocyte model system. J Biol Chem 276(51):48309–48317. https://doi.org/10.1074/jbc.M107146200
Xin W, Li X, Lu X, Niu K, Cai J (2011) Improved cardiac function after sarcoplasmic reticulum Ca(2+)-ATPase gene transfer in a heart failure model induced by chronic myocardial ischaemia. Acta Cardiol 66(1):57–64. https://doi.org/10.1080/AC.66.1.2064967
Xin W, Lu X, Li X, Niu K, Cai J (2011) Attenuation of endoplasmic reticulum stress-related myocardial apoptosis by SERCA2a gene delivery in ischemic heart disease. Mol Med 17(3–4):201–210. https://doi.org/10.2119/molmed.2010.00197
Bi X, Zhang G, Wang X, Nguyen C, May HI, Li X, Al-Hashimi AA, Austin RC, Gillette TG, Fu G, Wang ZV, Hill JA (2018) Endoplasmic reticulum chaperone GRP78 protects heart from ischemia/reperfusion injury through Akt activation. Circ Res 122(11):1545–1554. https://doi.org/10.1161/circresaha.117.312641
Yamashita K, Kajstura J, Discher DJ, Wasserlauf BJ, Bishopric NH, Anversa P, Webster KA (2001) Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res 88(6):609–614. https://doi.org/10.1161/01.res.88.6.609
Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A (2001) Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104(3):330–335. https://doi.org/10.1161/01.cir.104.3.330
Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101(6):660–667. https://doi.org/10.1161/01.cir.101.6.660
Ortega A, Roselló-Lletí E, Tarazón E, Molina-Navarro MM, Martínez-Dolz L, González-Juanatey JR, Lago F, Montoro-Mateos JD, Salvador A, Rivera M, Portolés M (2014) Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy. PLoS ONE 9(9):e107635. https://doi.org/10.1371/journal.pone.0107635
Kolattukudy PE, Niu J (2012) Inflammation, endoplasmic reticulum stress, autophagy, and the monocyte chemoattractant protein-1/CCR2 pathway. Circ Res 110(1):174–189. https://doi.org/10.1161/circresaha.111.243212
Ridker PM (2014) Inflammation, C-reactive protein, and cardiovascular disease: moving past the marker versus mediator debate. Circ Res 114:594–595. https://doi.org/10.1161/circresaha.114.303215
Marsh KG, Arrieta A, Thuerauf DJ, Blackwood EA, MacDonnell L, Glembotski CC (2021) The peroxisomal enzyme, FAR1, is induced during ER stress in an ATF6-dependent manner in cardiac myocytes. Am J Physiol Heart Circ Physiol 320(5):H1813–H1821. https://doi.org/10.1152/ajpheart.00999.2020
Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18(12):7499–7509. https://doi.org/10.1128/mcb.18.12.7499
Cui W, Li J, Ron D, Sha B (2011) The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallogr D Biol Crystallogr 67(Pt 5):423–428. https://doi.org/10.1107/s0907444911006445
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101(31):11269–11274. https://doi.org/10.1073/pnas.0400541101
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3):619–633. https://doi.org/10.1016/s1097-2765(03)00105-9
Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP (2004) Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J 23:169–179. https://doi.org/10.1038/sj.emboj.7600030
Szegezdi E, Duffy A, O’Mahoney ME, Logue SE, Mylotte LA, O’brien T, Samali A, (2006) ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun 349:1406–1411. https://doi.org/10.1016/j.bbrc.2006.09.009
Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3(6):a004317. https://doi.org/10.1101/cshperspect.a004317
Zeng Z, Huang N, Zhang Y, Wang Y, Su Y, Zhang H, An Y (2020) CTCF inhibits endoplasmic reticulum stress and apoptosis in cardiomyocytes by upregulating RYR2 via inhibiting S100A1. Life Sci 242:117158. https://doi.org/10.1016/j.lfs.2019.117158
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12(7):982–995. https://doi.org/10.1101/gad.12.7.982
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18(24):3066–3077. https://doi.org/10.1101/gad.1250704
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24(6):1243–1255. https://doi.org/10.1038/sj.emboj.7600596
Avery J, Etzion S, DeBosch BJ, Jin X, Lupu TS, Beitinjaneh B, Grand J, Kovacs A, Sambandam N, Muslin AJ (2010) TRB3 function in cardiac endoplasmic reticulum stress. Circ Res 106(9):1516–1523. https://doi.org/10.1161/circresaha.109.211920
Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M (2010) Biology of endoplasmic reticulum stress in the heart. Circ Res 107(10):1185–1197. https://doi.org/10.1161/circresaha.110.227033
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459. https://doi.org/10.1128/mcb.23.21.7448-7459.2003
Wang ZV, Deng Y, Gao N, Pedrozo Z, Li DL, Morales CR, Criollo A, Luo X, Tan W, Jiang N, Lehrman MA, Rothermel BA, Lee AH, Lavandero S, Mammen PPA, Ferdous A, Gillette TG, Scherer PE, Hill JA (2014) Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 156(6):1179–1192. https://doi.org/10.1016/j.cell.2014.01.014
Li J, Xie J, Wang YZ, Gan YR, Wei L, Ding GW, Ding YH, Xie DX (2021) Overexpression of lncRNA Dancr inhibits apoptosis and enhances autophagy to protect cardiomyocytes from endoplasmic reticulum stress injury via sponging microRNA-6324. Mol Med Rep 23(2):116. https://doi.org/10.3892/mmr.2020.11755
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666. https://doi.org/10.1126/science.287.5453.664
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16(11):1345–1355. https://doi.org/10.1101/gad.992302
Yamaguchi O, Higuchi Y, Hirotani S, Kashiwase K, Nakayama H, Hikoso S, Takeda T, Watanabe T, Asahi M, Taniike M, Matsumura Y, Tsujimoto I, Hongo K, Kusakari Y, Kurihara S, Nishida K, Ichijo H, Hori M, Otsu K (2003) Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100(26):15883–15888. https://doi.org/10.1073/pnas.2136717100
Sozen E, Yazgan B, Tok OE, Demirel T, Ercan F, Proto JD, Ozer NK (2020) Cholesterol induced autophagy via IRE1/JNK pathway promotes autophagic cell death in heart tissue. Metabolism 106:154205. https://doi.org/10.1016/j.metabol.2020.154205
Kaneko M, Niinuma Y, Nomura Y (2003) Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull 26:931–935. https://doi.org/10.1248/bpb.26.931
Janssens S, Pulendran B, Lambrecht BN (2014) Emerging functions of the unfolded protein response in immunity. Nat Immunol 15:910–919. https://doi.org/10.1038/ni.2991
Kim BJ, Ryu SW, Song BJ (2006) JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem 281:21256–21265. https://doi.org/10.1074/jbc.m510644200
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276(17):13935–13940. https://doi.org/10.1074/jbc.m010677200
Son SM, Byun J, Roh SE, Kim SJ, Mook-Jung I (2014) Reduced IRE1α mediates apoptotic cell death by disrupting calcium homeostasis via the InsP3 receptor. Cell Death Dis 5:e1188. https://doi.org/10.1038/cddis.2014.129
Hollien J, Weissman JS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104–107. https://doi.org/10.1126/science.1129631
Maurel M, Dejeans N, Taouji S, Chevet E, Grosset CF (2013) MicroRNA-1291-mediated silencing of IREalpha enhances glypican-3 expression. RNA 19:778–788. https://doi.org/10.1261/rna.036483.112
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279(44):45495–45502. https://doi.org/10.1074/jbc.m406933200
Li Y, Guo Y, Tang J, Jiang J, Chen Z (2014) New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 46(8):629–640. https://doi.org/10.1093/abbs/gmu048
Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y (2011) C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol 31(5):1124–1132. https://doi.org/10.1161/atvbaha.111.224519
Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M (2007) Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation 116(11):1226–1233. https://doi.org/10.1161/circulationaha.106.682054
Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H (2005) AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 25:9554–9575. https://doi.org/10.1128/mcb.25.21.9554-9575.2005
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349. https://doi.org/10.1016/j.cell.2007.04.027
Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao M, Liu D, Qiao L, Li N, Zheng J, Chen D (2014) CHOP mediates ASPP2-induced autophagic apoptosis in hepatoma cells by releasing Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2. Cell Death Dis 5(7):e1323. https://doi.org/10.1038/cddis.2014.276
Lewis A, Hayashi T, Su TP, Betenbaugh MJ (2014) Bcl-2 family in inter-organelle modulation of calcium signaling; roles in bioenergetics and cell survival. J Bioenerg Biomembr 46(1):1–15. https://doi.org/10.1007/s10863-013-9527-7
Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA (2006) Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281(23):16016–16024. https://doi.org/10.1074/jbc.m601299200
Burton TR, Gibson SB (2009) The role of Bcl-2 family member BNIP3 in cell death and disease: NIPping at the heels of cell death. Cell Death Differ 16(4):515–523. https://doi.org/10.1038/cdd.2008.185
Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim DH (2012) The chemical chaperone 4-phenylbutyric acid attenuates pressure-overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun 421(3):578–584. https://doi.org/10.1016/j.bbrc.2012.04.048
Rani S, Sreenivasaiah PK, Kim JO, Lee MY, Kang WS, Kim YS, Ahn Y, Park WJ, Cho C, Kim DH (2017) Tauroursodeoxycholic acid (TUDCA) attenuates pressure overload-induced cardiac remodeling by reducing endoplasmic reticulum stress. PLoS ONE 12(4):e0176071. https://doi.org/10.1371/journal.pone.0176071
Pires Da Silva J, Monceaux K, Guilbert A, Gressette M, Piquereau J, Novotova M, Ventura-Clapier R, Garnier A, Lemaire C (2020) SIRT1 Protects the Heart from ER Stress-Induced Injury by Promoting eEF2K/eEF2-Dependent Autophagy. Cells 9(2):426. https://doi.org/10.3390/cells9020426
Song XJ, Yang CY, Liu B, Wei Q, Korkor MT, Liu JY, Yang P (2011) Atorvastatin inhibits myocardial cell apoptosis in a rat model with post-myocardial infarction heart failure by downregulating ER stress response. Int J Med Sci 8:564–572. https://doi.org/10.7150/ijms.8.564
Sukumaran V, Watanabe K, Veeraveedu PT, Gurusamy N, Ma M, Thandavarayan RA, Lakshmanan AP, Yamaguchi K, Suzuki K, Kodama M (2011) Olmesartan, an AT1 antagonist, attenuates oxidative stress, endoplasmic reticulum stress and cardiac inflammatory mediators in rats with heart failure induced by experimental autoimmune myocarditis. Int J Biol Sci 7:154–167. https://doi.org/10.7150/ijbs.7.154
Yao T, Ying X, Zhao Y, Yuan A, He Q, Tong H, Ding S, Liu J, Peng X, Gao E, Pu J, He B (2015) Vitamin D receptor activation protects against myocardial reperfusion injurythrough inhibition of apoptosis and modulation of autophagy. Antioxid Redox Signal 22:633–650. https://doi.org/10.1089/ars.2014.5887
Tao J, Zhu W, Li Y, Xin P, Li J, Liu M, Li J, Redington AN, Wei M (2011) Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol 301:H1471–H1486. https://doi.org/10.1152/ajpheart.00097.2011
Kim S, Kim S, Hwang AR, Choi HC, Lee JY, Woo CH (2020) Apelin-13 Inhibits Methylglyoxal-Induced Unfolded Protein Responses and Endothelial Dysfunction via Regulating AMPK Pathway. Int J Mol Sci 21(11):4069. https://doi.org/10.3390/ijms21114069
Bretón-Romero R, Weisbrod RM, Feng B, Holbrook M, Ko D, Stathos MM, Zhang JY, Fetterman JL, Hamburg NM (2018) Liraglutide treatment reduces endothelial endoplasmic reticulum stress and insulin resistance in patients with diabetes mellitus. J Am Heart Assoc 7(18):e009379. https://doi.org/10.1161/jaha.118.009379
Caillard A, Sadoune M, Cescau A, Meddour M, Gandon M, Polidano E, Delcayre C, Da Silva K, Manivet P, Gomez AM, Cohen-Solal A, Vodovar N, Li Z, Mebazaa A, Samuel JL (2018) QSOX1, a novel actor of cardiac protection upon acute stress in mice. J Mol Cell Cardiol 119:75–86. https://doi.org/10.1016/j.yjmcc.2018.04.014
Li Z, Meng Z, Lu J, Chen FM, Wong WT, Tse G, Zheng C, Keung W, Tse K, Li RA, Jiang L, Yao X (2018) TRPV6 protects ER stress-induced apoptosis via ATF6α-TRPV6-JNK pathway in human embryonic stem cell-derived cardiomyocytes. J Mol Cell Cardiol 120:1–11. https://doi.org/10.1016/j.yjmcc.2018.05.008
Cong XQ, Piao MH, Li Y, Xie L, Liu Y (2016) Bis(maltolato)oxovanadium(IV) (BMOV) attenuates apoptosis in high glucose-treated cardiac cells and diabetic rat hearts by regulating the unfolded protein responses (UPRs). Biol Trace Elem Res 173(2):390–398. https://doi.org/10.1007/s12011-016-0668-5
Minamino T, Komuro I, Kitakaze M (2010) Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 107(9):1071–1082. https://doi.org/10.1161/circresaha.110.227819
Guan HS, Shangguan HJ, Shang Z, Yang L, Meng XM, Qiao SB (2011) Endoplasmic reticulum stress caused by left ventricular hypertrophy in rats: effects of telmisartan. Am J Med Sci 342:318–323. https://doi.org/10.1097/MA.0b013e3182112baf
Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, Yao X, Wang N, Huang Y (2014) Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arteriosclerosis Thromb Vasc Biol 34:830–836. https://doi.org/10.1161/atvbaha.113.301938
Ni L, Zhou C, Duan Q, Lv J, Fu X, Xia Y, Wang DW (2011) Beta-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS ONE 6:e27294. https://doi.org/10.1371/journal.pone.0027294
Fu M, Zhang J, Xu S, Pang Y, Liu N, Tang C (2001) Role of calcineurin in angiotensin II-induced cardiac myocyte hypertrophy of rats. Chin Med Sci J 16(1):1–4
Zhong J, Ouyang H, Zheng S, Guo Z, Chen Y, Zhong Y, Zhong W, Zuo L, Lu J (2020) The YAP/SERCA2a signaling pathway protects cardiomyocytes against reperfusion-induced apoptosis. Aging Albany NY 12(13):13618–13632
Rahate K, Bhatt LK, Prabhavalkar KS (2020) ERCA stimulation: a potential approach in therapeutics. Chem Biol Drug Des 95(1):5–15. https://doi.org/10.1111/cbdd.13620
Kong X, Liu H, He X, Sun Y, Ge W (2020) Unraveling the mystery of cold stress-induced myocardial injury. Front Physiol 11:580811. https://doi.org/10.3389/fphys.2020.580811
Devarakonda T, Mauro AG, Guzman G, Hovsepian S, Cain C, Das A, Praveen P, Hossain MA, Salloum FN (2020) B7–33, a functionally selective relaxin receptor 1 agonist, attenuates myocardial infarction-related adverse cardiac remodeling in mice. J Am Heart Assoc 9(8):e015748. https://doi.org/10.1161/JAHA.119.015748
Fu Z, Mui D, Zhu H, Zhang Y (2020) Exenatide inhibits NF-κB and attenuates ER stress in diabetic cardiomyocyte models. Aging Albany NY 12(9):8640–8651
Sun JL, Abd El-Aty AM, Jeong JH, Jung TW (2020) Ginsenoside Rb2 ameliorates LPS-induced inflammation and ER stress in HUVECs and THP-1 cells via the AMPK-mediated pathway. Am J Chin Med 48(4):967–985. https://doi.org/10.1142/S0192415X20500469
Gao G, Chen W, Yan M, Liu J, Luo H, Wang C, Yang P (2020) Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int J Mol Med 45(1):195–209. https://doi.org/10.3892/ijmm.2019.4407
Wang S, Wang Z, Fan Q, Guo J, Galli G, Du G, Wang X, Xiao W (2016) Ginkgolide K protects the heart against endoplasmic reticulum stress injury by activating the inositol-requiring enzyme 1α/X box-binding protein-1 pathway. Br J Pharmacol 173(15):2402–2418. https://doi.org/10.1111/bph.13516
Wang M, Meng XB, Yu YL, Sun GB, Xu XD, Zhang XP, Dong X, Ye JX, Xu HB, Sun YF, Sun XB (2014) Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis 19(12):1727–1735. https://doi.org/10.1007/s10495-014-1039-3
Li YP, Wang SL, Liu B, Tang L, Kuang RR, Wang XB, Zhao C, Song XD, Cao XM, Wu X, Yang PZ, Wang LZ, Chen AH (2016) Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol Sin 37(3):344–353. https://doi.org/10.1038/aps.2015.130
Lin Y, Zhu J, Zhang X, Wang J, Xiao W, Li B, Jin L, Lian J, Zhou L, Liu J (2016) Inhibition of cardiomyocytes hypertrophy by resveratrol is associated with amelioration of endoplasmic reticulum stress. Cell Physiol Biochem 39(2):780–789. https://doi.org/10.1159/000447788
Hubbard BP, Sinclair DA (2014) Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci 35:146–154. https://doi.org/10.1016/j.tips.2013.12.004
Lou Y, Wang Z, Xu Y, Zhou P, Cao J, Li Y, Chen Y, Sun J, Fu L (2015) Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int J Mol Med 36(3):873–880. https://doi.org/10.3892/ijmm.2015.2291
Shen M, Wang L, Yang G, Gao L, Wang B, Guo X, Zeng C, Xu Y, Shen L, Cheng K, Xia Y, Li X, Wang H, Fan L, Wang X (2014) Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS ONE 9(2):e88389. https://doi.org/10.1371/journal.pone.0088389
Zhao GL, Yu LM, Gao WL, Duan WX, Jiang B, Liu XD, Zhang B, Liu ZH, Zhai ME, Jin ZX, Yu SQ, Wang Y (2016) Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin 37(3):354–367. https://doi.org/10.1038/aps.2015.136
Jia LJ, Chen W, Shen H, Ji D, Zhao XM, Liu XH (2008) Effects of anisodamine on microcirculation of the asystole rats during the cardiopulmonary resuscitation. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 20(12):737–739
Yin XL, Shen H, Zhang W, Yang Y (2011) Inhibition of endoplasm reticulum stress by anisodamine protects against myocardial injury after cardiac arrest and resuscitation in rats. Am J Chin Med 39(5):853–866. https://doi.org/10.1142/s0192415x11009251
Wang L-C, Zhang W-S, Liu Q, Li J, Alolga R, Liu K, Liu B-L, Li P, Qi L-W (2015) A standardized notoginseng extract exerts cardioprotection by attenuating apoptosis under endoplasmic reticulum stress conditions. J Funct Foods 16:20–27. https://doi.org/10.1016/j.jff.2015.04.018
Liu M, Wang XR, Wang C, Song DD, Liu XH, Shi DZ (2013) Panax quinquefolium saponin attenuates ventricular remodeling after acute myocardial infarction by inhibiting chop-mediated apoptosis. Shock 40(4):339–344. https://doi.org/10.1097/shk.0b013e3182a3f9e5
Liu M, Wang XR, Wang C, Song DD, Liu XH, Shi DZ (2012) Panax quinquefolium saponins reduce myocardial hypoxia-reoxygenation injury by inhibiting excessive endoplasmic reticulum stress. Shock 37(2):228–233. https://doi.org/10.1097/shk.0b013e3182a3f9e5
Liu M, Xue M, Wang XR, Tao TQ, Xu FF, Liu XH, Shi DZ (2015) Panax quinquefolium saponin attenuates cardiomyocyte apoptosis induced by thapsigargin through inhibition of endoplasmic reticulum stress. J Geriatr Cardiol 12(5):540–546
Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang J, Ai Q, Xing N, Sun X (2016) Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep 6:21730. https://doi.org/10.1038/srep21730
Choy KW, Mustafa MR, Lau YS, Liu J, Murugan D, Lau CW, Wang L, Zhao L, Huang Y (2016) Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARdelta signaling pathway. Biochem Pharmacol 116:51–62. https://doi.org/10.1016/j.bcp.2016.07.013
Yu Y, Xing N, Xu X, Zhu Y, Wang S, Sun G, Sun X (2019) Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine 52:178–186. https://doi.org/10.1016/j.phymed.2018.09.168
Yang M, Mao G, Ouyang L, Shi C, Hu P, Huang S (2020) Crocetin alleviates myocardial ischemia/reperfusion injury by regulating inflammation and the unfolded protein response. Mol Med Rep 21(2):641–648. https://doi.org/10.3892/mmr.2019.10891
Wang X, Yuan B, Cheng B, Liu Y, Zhang B, Wang X, Lin X, Yang B, Gong G (2019) Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of miR-34a/Sirt1/Nrf2 pathway. Shock 51:123–130. https://doi.org/10.1097/shk.0000000000001116
Xu L, Deng Y, Feng L, Li D, Chen X, Ma C, Liu X, Yin J, Yang M, Teng F, Wu W, Guan S, Jiang B, Guo D (2011) Cardio-protection of salvianolic acid B through inhibition of apoptosis network. PLoS ONE 6(9):e24036. https://doi.org/10.1371/journal.pone.0024036
Chen R, Sun G, Yang L, Wang J, Sun X (2016) Salvianolic acid B protects against doxorubicin induced cardiac dysfunction via inhibition of ER stress mediated cardiomyocyte apoptosis. Toxicol Res (Camb) 5(5):1335–1345. https://doi.org/10.1039/c6tx00111d
Zhou X, Xin Q, Wang Y, Zhao Y, Chai H, Huang X, Tao X, Zhao M (2016) Total flavonoids of astragalus plays a cardioprotective role in viral myocarditis. Acta Cardiol Sin 32(1):81–88. https://doi.org/10.6515/acs20150424h
Pal R, Cristan EA, Schnittker K, Narayan M (2010) Rescue of ER oxidoreductase function through polyphenolic phytochemical intervention: implications for subcellular traffic and neurodegenerative disorders. Biochem Biophys Res Commun 392:567–571. https://doi.org/10.1016/j.bbrc.2010.01.071
Cheng WP, Wang BW, Shyu KG (2009) Regulation of GADD153 induced by mechanical stress in cardiomyocytes. Eur J Clin Invest 39:960–971. https://doi.org/10.1111/j.1365-2362.2009.02193.x
Chen J, Li L, Bai X, Xiao L, Shangguan J, Zhang W, Zhang X, Wang S, Liu G (2021) Inhibition of autophagy prevents panax notoginseng saponins (PNS) protection on cardiac myocytes against endoplasmic reticulum (ER) stress-induced mitochondrial injury, Ca2+ homeostasis and associated apoptosis. Front Pharmacol 12:620812. https://doi.org/10.3389/fphar.2021.620812
Wu Y, Cui H, Zhang Y, Yu P, Li Y, Wu D, Xue Y, Fu W (2021) Inonotus obliquus extract alleviates myocardial ischemia/reperfusion injury by suppressing endoplasmic reticulum stress. Mol Med Rep 23(1):77. https://doi.org/10.3892/mmr.2020.11716
Fan CL, Yao ZH, Ye MN, Fu LL, Zhu GN, Dai Y, Yao XS (2020) Fuziline alleviates isoproterenol-induced myocardial injury by inhibiting ROS-triggered endoplasmic reticulum stress via PERK/eIF2α/ATF4/Chop pathway. J Cell Mol Med 24(2):1332–1344. https://doi.org/10.1111/jcmm.14803
Wan YJ, Wang YH, Guo Q, Jiang Y, Tu PF, Zeng KW (2021) Protocatechualdehyde protects oxygen-glucose deprivation/reoxygenation-induced myocardial injury via inhibiting PERK/ATF6α/IRE1α pathway. Eur J Pharmacol 891:173723. https://doi.org/10.1016/j.ejphar.2020.173723
Zhao G, Zhang X, Wang H, Chen Z (2020) Beta carotene protects H9c2 cardiomyocytes from advanced glycation end product-induced endoplasmic reticulum stress, apoptosis, and autophagy via the PI3K/Akt/mTOR signaling pathway. Ann Transl Med 8(10):647
Zhang Q, Shi J, Guo D, Wang Q, Yang X, Lu W, Sun X, He H, Li N, Wang Y, Li C, Wang W (2020) Qishen Granule alleviates endoplasmic reticulum stress-induced myocardial apoptosis through IRE-1-CRYAB pathway in myocardial ischemia. J Ethnopharmacol 252:112573. https://doi.org/10.1016/j.jep.2020.112573
Acknowledgements
We thank the members of the Sengupta lab for review of the manuscript.
Funding
The work was supported by Central University Grant Commission-Start Up Grant [No. F.4.5(192-FRP)/2015(BSR)] to AS, project Grant sponsored by Department of Science and Technology to AS (DST-SERB, CRG/2020.000348) project Grant sponsored by Department of Science and Technology to SC (DST-SERB, No.: EMR/2017/001382).
Author information
Authors and Affiliations
Contributions
SD, AM, JS all reviewed the literature. AS and SC designed the review paper and SD wrote the manuscript. All authors have read the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, S., Mondal, A., Samanta, J. et al. Unfolded protein response during cardiovascular disorders: a tilt towards pro-survival and cellular homeostasis. Mol Cell Biochem 476, 4061–4080 (2021). https://doi.org/10.1007/s11010-021-04223-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04223-0