Abstract
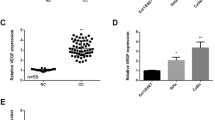
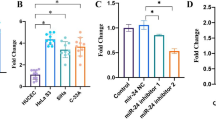
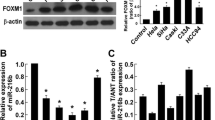
To explore the mechanism of miR-202-5p targeting the expression of PIK3CA and mediating the activation of PI3K/Akt/mTOR signaling pathway on the proliferation, invasion, and epithelial–mesenchymal transition (EMT) of cervical cancer. The objects of study were 105 cases of cervical cancer and their corresponding normal tissues. qRT-PCR was used to detect the expression of miR-202-5p and PIK3CA in adjacent normal tissue and cervical cancer tissue. Dual luciferase reporter assay was used to verify the targeting relationship between miR-202-5p and PIK3CA gene. Human cervical cancer cell lines HPV-16E6, SiHa, HeLa, and CaSki were purchased for our cell experiments. The expression levels of PIK3CA in the cells were detected by qRT-PCR. The cell line with higher expression levels was selected to complete the follow-up experiment. The cultured cells were transfected and divided into the miR-202-5p mimic NC group, miR-202-5p mimic group, miR-202-5p inhibitor NC group, miR-202-5p inhibitor group, siRNA-PIK3CA NC group, siRNA-PIK3CA group, miR-202-5p inhibitor NC + siRNA-PIK3CA NC group, miR-202-5p inhibitor + siRNA-PIK3CA NC group, and miR-202-5p inhibitor + siRNA-PIK3CA group. QRT-PCR was used to detect the expression of miR-202-5p. Western blot and qRT-PCR were applied to detect the mRNA and protein expression levels of related pathway proteins (PIK3CA, PI3K, PTEN, p-Akt1, and p-mTOR) and epithelial–mesenchymal transition-related factors (N-cadherin, E-cadherin, and vimentin). Cell proliferation was detected by plate colony formation assay. Transwell assay was used to detect the invasion ability of each group. When compared with the adjacent tissues, PIK3CA mRNA expression level was significantly increased and miR-202-5p expression level was significantly decreased in cervical cancer tissues (all P < 0.05). PIK3CA was a target gene of miR-202-5p. The mRNA expression level of PIK3CA in SiHa cervical cancer cells was significantly higher than that in CaSki, HeLa, and HPV-16E6 cells (all P < 0.05), and SiHa cervical cancer cells were selected to complete the follow-up experiments. When compared with the corresponding NC group, the expression of miR-202-5p in miR-202-5p mimic group was increased. In addition, the mRNA and protein expression levels of E-cadherin and PTEN in miR-202-5p mimic and siRNA-PIK3CA groups were increased, and the protein expression of p-Akt1 and p-mTOR was decreased, and also, the mRNA and protein expression levels of PIK3CA, PI3K, N-cadherin, and vimentin were decreased (all P < 0.05); in miR-202-5p inhibitor group, the expression levels of miR-202-5p, E-cadherin, and PTEN decreased, the protein expression of p-Akt1 and p-mTOR increased, and the mRNA and protein expression of PIK3CA, PI3K, N-cadherin, and vimentin increased in miR-202-5p inhibitor group (all P < 0.05); in miR-202-5p inhibitor + siRNA-PIK3CA group, the expression of miR-202-5p decreased (P < 0.05), but the mRNA and protein expression of PIK3CA, PI3K, p-Akt1, p-mTOR, N-cadherin, E-cadherin, and vimentin had no significant changes (all P > 0.05). When compared with the corresponding NC group, the number of cell clones in miR-202-5p mimic group and siRNA-PIK3CA group was decreased, and the invasion ability of miR-202-5p inhibitor group was increased, and the invasion ability was enhanced (all P < 0.05); miR-202-5p inhibitor + siRNA-PIK3CA group showed no significant change in the number of cell clones and the rate of invasion (P > 0.05). In conclusion, the overexpression of miR-202-5p can suppress PIK3CA gene expression and the activation of PI3K/Akt/mTOR signaling pathway to suppress the proliferation, invasion, and EMT of cervical cancer.







Similar content being viewed by others
Data availability
The datasets supporting the conclusions of this article are included within the article.
References
Shrestha AD, Neupane D, Vedsted P, Kallestrup P (2018) Cervical cancer prevalence, incidence and mortality in low and middle income countries: a systematic review. Asian Pac J Cancer Prev 19(2):319–324
Clifford GM, Tully S, Franceschi S (2017) Carcinogenicity of human papillomavirus (HPV) types in HIV-positive women: a meta-analysis from HPV infection to cervical cancer. Clin Infect Dis 64(9):1228–1235
Tao X, Austin RM, Zhang H, Zhang L, Xiao J, Wang L, Zhou X, Zhao C (2015) Pap test reporting rates for conventional smear and liquid-based cervical cytology from the largest academic women’s hospital in china: analysis of 1,248,785 pap test reports. Acta Cytol 59(6):445–451
Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, Bothou A, Galazios G (2016) Cervical cancer: screening, diagnosis and staging. J BUON 21(2):320–325
Hu Z, Ma D (2018) The precision prevention and therapy of HPV-related cervical cancer: new concepts and clinical implications. Cancer Med 7(10):5217–5236
US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr., Kemper AR, Kubik M, Landefeld CS, Mangione CM, Phipps MG, Silverstein M, Simon MA, Tseng CW, Wong JB (2018) Screening for cervical cancer: US preventive services task force recommendation statement. JAMA 320(7):674–686
Li H, Wu X, Cheng X (2016) Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol 27(4):e43
Chopra S, Gupta M, Mathew A, Mahantshetty U, Engineer R, Lavanya G, Gupta S, Ghosh J, Thakur M, Deodhar K, Menon S, Rekhi B, Bajpai J, Gulia S, Maheshwari A, Kerkar R, Shylasree TS, Shrivastava SK (2018) Locally advanced cervical cancer: a study of 5-year outcomes. Indian J Cancer 55(1):45–49
Kokka F, Bryant A, Brockbank E, Powell M, Oram D (2015) Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev 4:CD010260
Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, Mirzaei HR, Pourhanifeh MH, Bokharaei-Salim F, Mirzaei H, Hamblin MR (2020) Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: a review. Int J Cancer 146(2):305–320
Pardini B, De Maria D, Francavilla A, Di Gaetano C, Ronco G, Naccarati A (2018) MicroRNAs as markers of progression in cervical cancer: a systematic review. BMC Cancer 18(1):696
Gao L, Zhang LJ, Li SH, Wei LL, Luo B, He RQ, Xia S (2018) Role of miR-452-5p in the tumorigenesis of prostate cancer: a study based on the Cancer Genome Atl(TCGA), gene expression omnibus (GEO), and bioinformatics analysis. Pathol Res Pract 214(5):732–749
Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M (2019) Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci 20(24):6249
Huang Y (2018) The novel regulatory role of lncRNA-miRNA-mRNA axis in cardiovascular diseases. J Cell Mol Med 22(12):5768–5775
Akhtar MM, Micolucci L, Islam MS, Olivieri F, Procopio AD (2019) A practical guide to miRNA target prediction. Methods Mol Biol 1970:1–13
Asiaf A, Ahmad ST, Arjumand W, Zargar MA (2018) MicroRNAs in breast cancer: diagnostic and therapeutic potential. Methods Mol Biol 1699:23–43
Bertoli G, Cava C, Castiglioni I (2015) MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics 5(10):1122–1143
Svoronos AA, Engelman DM, Slack FJ (2016) OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res 76(13):3666–3670
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16(3):203–222
Tutar Y (2014) miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol 15(5):429
Mishra S, Yadav T, Rani V (2016) Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol 98:12–23
Wang L, Zhao Y, Xiong W, Ye W, Zhao W, Hua Y (2019) MicroRNA-449a is downregulated in cervical cancer and inhibits proliferation, migration, and invasion. Oncol Res Treat 42(11):564–571
Zhong J, Yuan H, Xu X, Kong S (2018) MicroRNA-195 inhibits cell proliferation, migration and invasion by targeting defective in cullin neddylation 1 domain containing 1 in cervical cancer. Int J Mol Med 42(2):779–788
Zhang Z, Wang J, Wang X, Song W, Shi Y, Zhang L (2018) MicroRNA-21 promotes proliferation, migration, and invasion of cervical cancer through targeting TIMP3. Arch Gynecol Obstet 297(2):433–442
Zhang S, Cai J, Xie W, Luo H, Yang F (2018) miR-202 suppresses prostate cancer growth and metastasis by targeting PIK3CA. Exp Ther Med 16(2):1499–1504
Wang J, Chen J, Sun F, Wang Z, Xu W, Yu Y, Ding F, Shen H (2020) miR-202 functions as a tumor suppressor in hepatocellular carcinoma by targeting HK2. Oncol Lett 19(3):2265–2271
Gao S, Cao C, Dai Q, Chen J, Tu J (2018) miR-202 acts as a potential tumor suppressor in breast cancer. Oncol Lett 16(1):1155–1162
Dou D, Shi YF, Liu Q, Luo J, Liu JX, Liu M, Liu YY, Li YL, Qiu XD, Tan HY (2018) Hsa-miR-202-3p, up-regulated in type 1 gastric neuroendocrine neoplasms, may target DUSP1. World J Gastroenterol 24(5):573–582
Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F (2020) Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 8(2):e191–e203
Leite KRM, Pimenta R, Canavez J, Canavez F, de Souza FR, Vara L, Estivallet C, Camara-Lopes LH (2020) HPV genotype prevalence and success of vaccination to prevent cervical cancer. Acta Cytol 64(5):420–424
Ediriweera MK, Tennekoon KH, Samarakoon SR (2019) Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin Cancer Biol 59:147–160
Polivka J Jr., Janku F (2014) Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther 142(2):164–175
Duarte A, Silveira GG, Soave DF, Costa JPO, Silva AR (2019) The role of the LY294002—a non-selective inhibitor of phosphatidylinositol 3-kinase (PI3K) pathway- in cell survival and proliferation in cell line SCC-25. Asian Pac J Cancer Prev 20(11):3377–3383
Bishop JD, Nien WL, Dauphinee SM, Too CK (2006) Prolactin activates mammalian target-of-rapamycin through phosphatidylinositol 3-kinase and stimulates phosphorylation of p70S6K and 4E-binding protein-1 in lymphoma cells. J Endocrinol 190(2):307–312
Li X, Dai J, Ni D, He X, Zhang H, Zhang J, Fu Q, Liu Y, Lu S (2020) Insight into the mechanism of allosteric activation of PI3Kα by oncoprotein K-Ras4B. Int J Biol Macromol 1(144):643–655
Zhang B, Song Y, Sun S, Han R, Hua C, van der Veen S, Cheng H (2019) Human Papillomavirus 11 early protein E6 activates autophagy by repressing AKT/mTOR and Erk/mTOR. J Virol 93(12):e00172-e219
Deng W, Han W, Fan T, Wang X, Cheng Z, Wan B, Chen J (2018) Scutellarin inhibits human renal cancer cell proliferation and migration via upregulation of PTEN. Biomed Pharmacother 107:1505–1513
Ligresti G, Militello L, Steelman LS, Cavallaro A, Basile F, Nicoletti F, Stivala F, McCubrey JA, Libra M (2009) PIK3CA mutations in human solid tumors: role in sensitivity to various therapeutic approaches. Cell Cycle 8(9):1352–1358
Arafeh R, Samuels Y (2019) PIK3CA in cancer: the past 30 years. Semin Cancer Biol 59:36–49
Chen L, Yang L, Yao L, Kuang XY, Zuo WJ, Li S, Qiao F, Liu YR, Cao ZG, Zhou SL, Zhou XY, Yang WT, Shi JX, Huang W, Hu X, Shao ZM (2018) Characterization of PIK3CA and PIK3R1 somatic mutations in Chinese breast cancer patients. Nat Commun 9(1):1357
Ranieri C, Di Tommaso S, Loconte DC, Grossi V, Sanese P, Bagnulo R, Susca FC, Forte G, Peserico A, De Luisi A, Bartuli A, Selicorni A, Melis D, Lerone M, Praticò AD, Abbadessa G, Yu Y, Schwartz B, Ruggieri M, Simone C, Resta N (2018) In vitro efficacy of ARQ 092, an allosteric AKT inhibitor, on primary fibroblast cells derived from patients with PIK3CA-related overgrowth spectrum (PROS). Neurogenetics 19(2):77–91
Lachkar B, Minaguchi T, Akiyama A, Liu S, Zhang S, Xu C, Shikama A, Tasaka N, Sakurai M, Nakao S, Ochi H, Yoshikawa H, Satoh T (2018) Prognostic significance of PIK3CA mutation in stage IIB to IVA cervical cancers treated by concurrent chemoradiotherapy with weekly cisplatin. Medicine (Baltimore) 97(31):e11392
Krishnamurthy S, Yoda H, Hiraoka K, Inoue T, Lin J, Shinozaki Y, Watanabe T, Koshikawa N, Takatori A, Nagase H (2021) Targeting the mutant PIK3CA gene by DNA-alkylating pyrrole-imidazole polyamide in cervical cancer. Cancer Sci 112(3):1141–1149
Wu Y, Li X, Yu J, Björkholm M, Xu D (2019) ASF1a inhibition induces p53-dependent growth arrest and senescence of cancer cells. Cell Death Dis 10(2):76
Guo F, Zhang H, Jia Z, Cui M, Tian J (2018) Chemoresistance and targeting of growth factors/cytokines signalling pathways: towards the development of effective therapeutic strategy for endometrial cancer. Am J Cancer Res 8(7):1317–1331
Yang W, Feng B, Meng Y, Wang J, Geng B, Cui Q, Zhang H, Yang Y, Yang J (2019) FAM3C-YY1 axis is essential for TGFβ-promoted proliferation and migration of human breast cancer MDA-MB-231 cells via the activation of HSF1. J Cell Mol Med 23(5):3464–3475
Wan L, Pantel K, Kang Y (2013) Tumor metastasis: moving new biological insights into the clinic. Nat Med 19(11):1450–1464
Smeda M, Kieronska A, Adamski MG, Proniewski B, Sternak M, Mohaissen T, Przyborowski K, Derszniak K, Kaczor D, Stojak M, Buczek E, Jasztal A, Wietrzyk J, Chlopicki S (2018) Nitric oxide deficiency and endothelial-mesenchymal transition of pulmonary endothelium in the progression of 4T1 metastatic breast cancer in mice. Breast Cancer Res 20(1):86
Gurzu S, Kobori L, Fodor D, Jung I (2019) Epithelial–mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. Biomed Res Int 29(2019):2962580
Caccuri F, Ronca R, Laimbacher AS, Berenzi A, Steimberg N, Campilongo F, Mazzuca P, Giacomini A, Mazzoleni G, Benetti A, Caselli E, Presta M, Di Luca D, Fraefel C, Caruso A (2017) U94 of human herpesvirus 6 down-modulates Src, promotes a partial mesenchymal-to-epithelial transition and inhibits tumor cell growth, invasion and metastasis. Oncotarget 8(27):44533–44549
Zhang L, Xu J, Yang G, Li H, Guo X (2018) miR-202 inhibits cell proliferation, migration, and invasion by targeting epidermal growth factor receptor in human bladder cancer. Oncol Res 26(6):949–957
Ding Q, Jin M, Wang Y, Liu J, Kalds P, Wang Y, Yang Y, Wang X, Chen Y (2020) Transactivation of miR-202-5p by Steroidogenic Factor 1 (SF1) induces apoptosis in goat granulosa cells by targeting TGFβR2. Cells 9(2):445
Li C, Ma D, Yang J, Lin X, Chen B (2018) miR-202-5p inhibits the migration and invasion of osteosarcoma cells by targeting ROCK1. Oncol Lett 16(1):829–834
Yu HY, Pan SS (2020) MiR-202-5p suppressed cell proliferation, migration and invasion in ovarian cancer via regulating HOXB2. Eur Rev Med Pharmacol Sci 24(5):2256–2263
Liu T, Guo J, Zhang X (2019) MiR-202-5p/PTEN mediates doxorubicin-resistance of breast cancer cells via PI3K/Akt signaling pathway. Cancer Biol Ther 20(7):989–998
Dai B, Yu R, Fan M, Yang T, Wang B, Zhang Y (2019) HMQ-T-F2 suppresses migration of the human cervical cancer HeLa cells by reversing EMT via the PI3K/Akt signaling pathway. Oncol Rep 42(4):1451–1458
Acknowledgements
No.
Funding
No.
Author information
Authors and Affiliations
Contributions
YZ, LX, SX, WY, HZ, YM, JL, and XW designed the study, performed the study, analyzed the results and wrote the manuscript. All authors revised the manuscript and agreed to be accountable for all aspects of the presented work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent for publication
All authors have read and agreed to the published version of the manuscript.
Ethical approval
The study was approved by the clinical experimental ethics committee of the First Hospital of Hebei Medical University. All patients had informed consent to the experiment and signed the informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zheng, Y., Xie, L., Xu, S. et al. Effects of miR-202-5p silencing PIK3CA gene expression on proliferation, invasion, and epithelial–mesenchymal transition of cervical cancer SiHa cells through inhibiting PI3K/Akt/mTOR signaling pathway activation. Mol Cell Biochem 476, 4031–4044 (2021). https://doi.org/10.1007/s11010-021-04211-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04211-4