Abstract
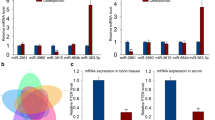
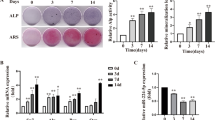
Osteoporosis is a common skeletal disease characterized by reduced bone mass partially caused by an imbalance between bone resorption and formation. Considering the potential role of microRNAs (miRNAs) in osteoporosis, we attempted to identify deregulated miRNA that participates in the pathogenesis of osteoporosis. We analyzed online datasets for differentially expressed miRNAs and predicted deregulated miRNA target genes, applied these genes for signaling pathway enrichment annotation, and selected the possible miR-99b-5p/FGFR3 axis. Within osteoporosis bone tissues, miR-99b-5p was upregulated and FGFR3 was downregulated. miR-99b-5p overexpression inhibited osteoblast proliferation and osteogenesis-related genes expression, whereas FGFR3 overexpression exerted opposite effects upon the proliferation of osteoblasts and osteogenesis-related genes expression. By direct targeting, miR-99b-5p inhibited FGFR3 expression. Moreover, FGFR3 silencing significantly reversed the roles of miR-99b-5p inhibition in the proliferation of osteoblasts and osteogenesis-related genes expression. In conclusion, we identify a deregulated miRNA/mRNA axis in osteoporosis and osteogenic differentiation, namely the miR-99b-5p/FGFR3 axis; through targeting FGFR3, miR-99b-5p inhibits osteoblast proliferation and activity, which might subsequently affect the bone formation in osteoporosis progression.






Similar content being viewed by others
References
Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–50.
Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2:90–6.
Khosla S, Riggs BL. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin N Am. 2005;34:1015–30, xi.
Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2011;13:27–38.
Bianco P, Sacchetti B, Riminucci M. Stem cells in skeletal physiology and endocrine diseases of bone. Endocr Dev. 2011;21:91–101.
Karsenty G. Minireview: transcriptional control of osteoblast differentiation. Endocrinology. 2001;142:2731–3.
Lian JB, Stein GS, Javed A, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16.
Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473:98–105.
Marie PJ. Cellular and molecular alterations of osteoblasts in human disorders of bone formation. Histol Histopathol. 1999;14:525–38.
Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37.
Marie PJ. Human osteoblastic cells: a potential tool to assess the etiology of pathologic bone formation. J Bone Miner Res. 1994;9:1847–50.
Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5.
Kosik KS. MicroRNAs and cellular phenotypy. Cell. 2010;143:21–6.
Burke M. Feds: provider capacity critical to Oregon’s success. Hospitals. 1991;65:68–9.
De-Ugarte L, Yoskovitz G, Balcells S, et al. MiRNA profiling of whole trabecular bone: identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genom. 2015;8:75.
Kong Y, Nie ZK, Li F, Guo HM, Yang XL, Ding SF. MiR-320a was highly expressed in postmenopausal osteoporosis and acts as a negative regulator in MC3T3E1 cells by reducing MAP9 and inhibiting PI3K/AKT signaling pathway. Exp Mol Pathol. 2019;110:104282.
Li K, Chen S, Cai P, et al. MiRNA-483-5p is involved in the pathogenesis of osteoporosis by promoting osteoclast differentiation. Mol Cell Probes. 2020;49:101479.
Cheng F, Yang MM, Yang RH. MiRNA-365a-3p promotes the progression of osteoporosis by inhibiting osteogenic differentiation via targeting RUNX2. Eur Rev Med Pharmacol Sci. 2019;23:7766–74.
Han Y, Zhang K, Hong Y, et al. miR-342-3p promotes osteogenic differentiation via targeting ATF3. FEBS Lett. 2018;592:4051–65.
Qin D, Zhang H, Zhang H, Sun T, Zhao H, Lee WH. Anti-osteoporosis effects of osteoking via reducing reactive oxygen species. J Ethnopharmacol. 2019;244:112045.
Osawa Y, Matsushita M, Hasegawa S, et al. Activated FGFR3 promotes bone formation via accelerating endochondral ossification in mouse model of distraction osteogenesis. Bone. 2017;105:42–9.
Kawane T, Qin X, Jiang Q, et al. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci Rep. 2018;8:13551.
Fromigue O, Modrowski D, Marie PJ. Growth factors and bone formation in osteoporosis: roles for fibroblast growth factor and transforming growth factor beta. Curr Pharm Des. 2004;10:2593–603.
Hoogwerf BJ. Renin-angiotensin system blockade and cardiovascular and renal protection. Am J Cardiol. 2010;105:30A-A35.
Zhang FY, Yang FJ, Yang JL, Wang L, Zhang Y. Renin inhibition improves ovariectomy-induced osteoporosis of lumbar vertebra in mice. Biol Pharm Bull. 2014;37:1994–7.
Queiroz-Junior CM, Santos A, Galvao I, et al. The angiotensin converting enzyme 2/angiotensin-(1–7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone. 2019;128:115041.
Wang Z, Zhao Z, Yang Y, et al. MiR-99b-5p and miR-203a-3p function as tumor suppressors by targeting IGF-1R in gastric cancer. Sci Rep. 2018;8:10119.
Li W, Chang J, Wang S, et al. miRNA-99b-5p suppresses liver metastasis of colorectal cancer by down-regulating mTOR. Oncotarget. 2015;6:24448–62.
Liu R, Chen Y, Shou T, Hu J, Qing C. miRNA-99b-5p targets FZD8 to inhibit non-small cell lung cancer proliferation, migration and invasion. Onco Targets Ther. 2019;12:2615–21.
Li YJ, Wang Y, Wang YY. MicroRNA-99b suppresses human cervical cancer cell activity by inhibiting the PI3K/AKT/mTOR signaling pathway. J Cell Physiol. 2019;234:9577–91.
Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34:280–300.
Dambroise E, Ktorza I, Brombin A, et al. Fgfr3 is a positive regulator of osteoblast expansion and differentiation during zebrafish skull vault development. J Bone Miner Res. 2020;35(9):1782–97.
Haupt LM, Murali S, Mun FK, et al. The heparan sulfate proteoglycan (HSPG) glypican-3 mediates commitment of MC3T3-E1 cells toward osteogenesis. J Cell Physiol. 2009;220:780–91.
Acknowledgements
None.
Funding
This study was supported by the fund of Technological Innovation Guidance Plan of Science and Technology Department of Hunan Province (2018SK52507).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
The investigation was performed with the approval of the Research Ethics Committee of the Second Xiangya Hospital (2020 (Yan110)).
Informed consent
The informed consents were obtained from all patient enrolled.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, M., Liu, B., Chen, X. et al. MiR-99b-5p suppressed proliferation of human osteoblasts by targeting FGFR3 in osteoporosis. Human Cell 34, 1398–1409 (2021). https://doi.org/10.1007/s13577-021-00567-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00567-3