Abstract
Osteoporosis is detrimental to the health of skeletal structure and significantly increases the risks of bone fracture. Moreover, bone regeneration is adversely impaired by increased osteoclastic activities as a result of osteoporosis. In this study, we developed a novel formulation of injectable bone cement based on calcium phosphate silicate cement (CPSC) and leuprolide acetate (LA). Several combinations of LA-CPSC bone cement were characterized and, it is found that LA could increase the setting time and compressive strength of CPSC in a concentration-dependent manner. Moreover, the in vitro results revealed that LA-CPSC was biocompatible and able to encourage the osteoblast proliferation via the mTOR signalling pathway. Furthermore, the LA-CPSC was implanted in the osteoporotic rats to evaluate its effectiveness to repair bone fractures under the osteoporotic conditions. The biomarker study and micro-CT analyses indicated that LA-CPSC could effectively reduce the osteoclast activities and promote the bone regeneration. In conclusion, our study demonstrated that LA-CPSC injectable bone cement should be a viable solution to repair bone fractures under the osteoporotic conditions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Osteoporosis (OP) is an age-related skeletal disease and characterized by abnormal bone turnover rate and excessive deterioration of bone microstructure. The infection of OP affects millions of elderly people in China and places a growing economic burden on the public health system [1]. In addition, the development of OP leads to fragility fractures, esp. in hips, and results in morbidities and mortalities in the elderly [2]. The development of OP can be attributed to many risk factors and the changes in the hormone level are believed to be closely related to the excessive bone resorption [3].
During the perimenopausal and postmenopausal periods, the estrogen level decreases dramatically and the osteoclast activity increases as a result of the diminishing suppressive effects of estrogen on the bone receptors. In recent studies, Sun et al found that the estrogen deficiency was not necessarily responsible for the development of OP but the high follicle stimulating hormone (FSH) level is strongly correlated to the excessive bone loss during these periods [4–6]. It is showed that FSH ligands can bind to the receptors (FSHRs) on osteoclast cells via the hormone-specific β subunit and activate osteoclastogenesis via the MEK/Erk, NF-κB and Akt signalling pathways [6–8]. In addition, FSH ligands induce immune and osteoblast cells to increase the synthesis of cytokines and RANK ligands, respectively, which subsequently result in the maturation of osteoclast cells [9]. Therefore, the treatment with the FSH antagonist may be an effective management of OP.
Leuprolide acetate (LA) is a member of the gonadotropin releasing hormone (GnRH) agonists and a continuous administration of LA is showed to significantly reduce the circulating levels of gonadotropins, such as the luteinizing hormone and FSH [10]. GnRH, or its agonists, acts on the receptors expressed on the anterior pituitary gonadotropes and regulates the syntheses of gonadotropins. The continuous administration of LA can desensitize the GnRH receptors and, hence decrease the release of FSH. Although several clinical studies found that the long-term administration of LA can result in the significant bone density loss, a controversial study showed that the low-dosage administration of LA might reduce the bone turnover rate and decrease the osteoclast cell activities in osteoporotic rats [11]. To our knowledge, there is no study yet attempting to blend LA with the injectable bone cement to repair bone fractures under the osteoporotic conditions.
Calcium phosphate silicate cement (CPSC) is a new generation of injectable bone cement and showed to be osteoinductive and osteoconductive. It can fit irregular shapes of bone fracture via injection and the in situ setting of CPSC can reinforce the fractured bones [12]. Upon degradation, CPSC releases abundant Ca2+, PO4 3− and SiO4 4− ions, which stimulate the regeneration of native bone tissues [13]. In addition, it can be loaded with different pharmaceuticals as local drug delivery devices to treat various bone diseases [14–16]. In this present study, LA was blended with CPSC in different concentrations and the impacts of LA on CPSC material properties were characterized and investigated. The biocompatibility of the LA-loaded CPSC was also evaluated based on its cytotoxicity to osteoblast cells and the regulation on gene expressions via the mammalian target of rapamycin (mTOR) signalling pathway. Furthermore, osteoporotic bone fractural models were created in the ovariectomized rats and the LA-loaded CPSC was implanted to observe its effectiveness in the bone regeneration under the osteoporotic conditions.
2. Material and methods
2.1. LA-CPSC slurry preparation
Dicalcium silicate ((CaO)2SiO2, C2S) and tricalcium silicate ((CaO)3SiO2, C3S) were synthesized by the sol–gel method as previously described [17]. Calcium silicate powders containing equal amounts of C2S and C3S were thoroughly blended with monocalcium phosphate (Ca(H2PO4)2, MCP, Sigma-Aldrich, China) in a planetary ball mill (YXQM-2L, MITR, China) for 4 h to prepare the homogeneous CPSC (C2S:C3S:MCP = 6.2:6.2:1 in weight ratio). LA (98% purity, ISUNPARM, Guangzhou, China) of different concentrations (0.4%, 0.8%, 1.0% and 1.5%) was blended in the CPSC and deionized water (ddH2O) was added to initiate the hydration process of the CPSC (CPSC:ddH2O = 2:1 in weight ratio). The resultant slurries were named as CPSC (LA-free), CPSC-4LA (0.4% LA), CPSC-8LA (0.8% LA), CPSC-10LA (1.0% LA) and CPSC-15LA (1.5% LA), respectively.
2.2. LA-CPSC slurry characterization
The setting times of different LA-CPSC slurries were measured in accordance with the ASTM C191-08 Standard and only the final setting times were recorded. In brief, the slurries were casted into polystyrene molds (φ = 17 mm; h = 2 mm). Every 5 min, the slurries were placed under the Vicat needle apparatus. Subsequently, the Vicat needle (φ = 1 mm and 8 g) was rested on the surface of slurry sample for 1–2 s and lifted. The slurries were considered set (hardened) when no visible mark of the Vicat needle was impressed on the LA-CPSC surfaces and the elapsed times since the initial contact between LA-CPSC and ddH2O were recorded as the final setting times. There were four replicates for each LA-CPSC formulation (n = 4).
The compressive strengths of hardened LA-CPSC were performed on the universal testing system (UTS, Instron 5944, U.S.A.). In brief, the slurries were casted into the polystyrene-based cylindrical molds (φ = 6 mm; h = 12 mm) and placed in the 100% relative humidity incubator for 3 and 7 d at 37 °C. Subsequently, the hardened samples were removed and the compressive strengths were examined on the UTS equipped with a 2 kN load cell at a crosshead speed of 1 mm min−1. There were four replicates for each LA-CPSC formulation (n = 4).
The porosities of LA-CPSC samples were measured by the Archimedes method. In brief, the LA-CPSC samples after 7 d hydration were dried in air and weighted to record the dry weight (Wdry). Subsequently, the samples were placed in anhydrous ethanol under vacuum for 4 h and the weights of samples saturated with ethanol were recorded as Wwet. Finally, the samples were placed in ethanol again and the weights of samples in ethanol were recorded as WEtOH. The porosities of LA-CPSC samples were calculated in the following equation: porosity (%) = (Wwet − Wdry)/(Wwet − WEtOH) × 100%. There were three replicates for each LA-CPSC formulation (n = 3).
The x-ray diffraction (XRD) and scanning electron microscope (SEM) analyses were performed to evaluate the LA impacts on the CPSC crystallinity and microstructure, respectively. In brief, the LA-CPSC samples were hydrated for 3 and 7 d and then ground into fine powders (particles size < 10 μm) in the planetary ball mill. The x-ray diffractometer (Empyrean, PANalytical, Netherlands; source Cu-Ka at 40 kV at 20 mA) was used to detect the XRD pattern of each LA-CPSC sample and the XRD scans were conducted between 10° and 60° at a 2° min−1 speed. The XRD patterns of LA-CPSC samples were subsequently processed and analyzed by JADE software (MDI, U.S.A.) equipped with the IODD PDF-4 database. In the SEM analyses, the LA-CPSC powders were sputter-coated with gold and observed in the SEM (20 kV and 100–110 μA, Hitachi S-3400N, Tokyo, Japan).
2.3. Osteoblast cell isolation
Osteoblast cells were isolated from calvaria of neonatal (<2 d old) Sprague–Dawley (SD) rats by an enzymatic digestive process as previously described and only osteoblast cells between the second and fourth passages were used in the following in vitro studies [18]. In brief, the rat calvaria fragments were washed three times with phosphate buffer saline (PBS, pH = 7.4) and digested in the 0.25% trypsin–EDTA solution at 37 °C for 45 min. Osteoblast cells were released from the calvaria in 1 mg ml−1 collagenase II (Gibco, U.S.A.) solution at 37 °C for 90 min and collected by centrifugation at 3000 rpm for 5 min. The cells were subsequently incubated in T25 culture flasks (ThermoFisher, U.S.A.) containing 10 ml of 10% fetal bovine serum (FBS, Gibco, U.S.A.)-Dulbecco's modified eagle's medium (DMEM, Sigma-Aldrich, U.S.A.) culture medium (referred to as the normal culture medium) at 37 °C and 5% CO2. The culture medium was replaced every 2 d.
2.4. LA-CPSC biocompatibility analyses
The biocompatibility of LA-CPSC was first evaluated based on the cytotoxicity of LA-CPSC extract to the osteoblast cells in accordance with the ISO 10993-5:2009 standard. In brief, the LA-CPSC samples after 7 d of hydration were first placed in 10 ml of PBS at 37 °C for 3 and 7 d and removed. The leftover PBS solutions were called LA-CPSC extracts and sterilized by filtering through a 0.22 µm membrane. Subsequently, the LA-CPSC extracts were mixed with the normal culture medium (extract:medium = 1:9) to prepare the extract culture media. To evaluate the cytotoxicity of extract culture medium, the osteoblast cells were seeded in a 96-well culture plate at a density of 5 × 103 cells/well and 100 μl of the extract culture medium was added into each well. Thereafter, the cell-seeded plate was incubated at 37 °C and 5% CO2 for 1 d and the cells incubated with the CPSC extract culture medium served as the control group. The optical density (OD) of viable cells was determined with the help of CCK-8 assay (Dojindo, Japan) by the microplate reader (CLARIOstar, BMG, Germany) at 450 nm and the relative cell viability was calculated as ODLA-CPSC/ODcontrol × 100%. Each group had three replicates (n = 3).
The biocompatibility of LA-CPSC was further investigated by the effects of extract culture media (CPSC-4LA, 7 d extract) on the osteoblastic gene expressions via the mTOR signalling pathway. In brief, the osteoblast cells were incubated with the extract culture media at 37 °C for 1 d for total RNA extraction. Total RNA was isolated via an RNA isolation kit (RNeasy Mini Kit, Qiagen, Germany) and reversely transcribed into complementary DNA. The cells cocultured with the CPSC extract culture medium served as the control group. In the first step of PCR analyses, 84 genes related to the mTOR signalling pathway were examined based on the commercial gene arrays (RT2 Profiler PCR Array, Qiagen, Germany) according to the manufacturer's instruction and each group had three replicates (n = 3). In the second step of PCR analyses, four genes (akt1, eif4b, rps6ka1 and rps6kb2, the primer sequences are presented in table 1) related to the mTOR pathway were further analyzed in the real-time PCR machine (CFX Connect, Bio-Rad, U.S.A.) in the following sequence: 30 s pre-denaturation at 95 °C, 5 s denaturation at 95 °C, 30 s annealing at 60 °C, repeating 40 cycles. Each group included three technical replicates (n = 3). The β-actin gene was used as the housekeeping gene and, in both PCR analyses, the cells incubated with the CPSC (without LA) extract culture medium served as the control group.
Table 1. The gene sequences of forward (f) and reverse (r) primer.
| Gene name | Direction | Primer sequence | Amplified fragment length (bp) |
|---|---|---|---|
| β-actin | f | CCTAAGGCCAACCGTGAAAA | 103 |
| r | CAGAGGCATACAGGGACAACAC | ||
| rps6ka1 | f | CAGACCGAGGGCAAGCTCTA | 124 |
| r | GTGCCAGCTCAGCCAGGTAA | ||
| akt1 | f | ATGGACTTCCGGTCAGGTTCA | 126 |
| r | GCCCTTGCCCAGTAGCTTCA | ||
| rps6kb2 | f | CTACTGAGTGTTCTGGGCAAGG | 127 |
| r | TGGCACTGCATACAATCTTGG | ||
| eif4b | f | TCCTGCCACAGACAGCTTTGA | 124 |
| r | GTCCCGATATCCGTCCCTATACC |
2.5. Osteoporotic bone fracture model preparation
Forty-eight female SD rats were used in this animal study to evaluate the effects of LA-CPSC on bone regeneration under the osteoporotic conditions. This animal study was approved by the ethic committee and conducted in compliance with Guidelines of Humane Treatment of Laboratory Animals of China (Permit No. G2008B0010). In brief, the rats (female, 6 ∼ 8 months old and 220 ± 20 g) were acclimated for 1 week prior to OP induction. The rats were anesthetized by the intraperitoneal injections of 10% chloral hydrate (0.3 mg g−1, Tianjin, China) and bilaterally ovariectomized. Subsequently, the rats, after 1 week of recovery, began to receive the twice-a-week subcutaneous injections of dexamethasone (2 × 10−3 mg g−1, Sigma-Aldrich, U.S.A.) for 3 months. Before the ovariectomy and after the 3 month injection of dexamethasone, 2 ml of blood was collected from each rat and stored at −80 °C for the biomarker analysis.
After the OP induction, the rats were randomly divided into four groups, which are the sham, CPSC, CPSC-4LA and CPSC-15LA groups. In the sham group, a bone fracture of 1.8 × 2 mm (diameter × depth) was perforated in the epiphysis of the left tibia of each rat by a blunt manual drill. This group also served as the control group. In the CPSC, CPSC-4LA and CPSC-15LA groups, the pastes of CPSC, CPSC-4LA and CPSC-15LA were placed in the bone perforations. In all groups, the incisions were closed by sutures and one week of antibiotic injection was given to each modelled animal to prevent inflammation. At the predefined endpoints (the 2nd, 4th and 6th week after perforation or implantation), 2 ml of blood was collected from each modelled animal and stored at −80 °C for the biomarker analysis. Subsequently, the modelled rats were euthanized by the injection of excessive amount of 10% chloral hydrate and the epiphyses of left tibiae were excised for the micro-CT analysis. There were four biological replicates for each group at each endpoint (n = 4).
2.6. The biomarker and micro-CT analyses
In this study, only one biomarker, tartrate resistant acid phosphatase 5b (TRACP-5b), from serum was evaluated by the enzyme-linked immunosorbent assay (ELISA) method. This ELISA kit was purchased from IBL company (Germany). In brief, the blood samples stored at −80 °C were thawed at room temperature and centrifuged at 6000 rpm for 5 min to isolate the serums. Subsequently, drops of 10 μl serum were transferred to the 96-well plate provided in the ELISA kit and the reagents were added into each well according to the manufactural instructions. The plate was incubated at 37 °C from 10 min away from light and read in the microplate reader (CLARIOstar, BMG, Germany) at the wavelength of 450 nm. In the biomarker analysis, there were three technical replicates for each serum sample.
In the micro-CT analysis, excised epiphyses were first rinsed with PBS thoroughly to remove residual soft tissues and then fixed in 10% neutral formalin solution (pH = 7.4) for 10 d. Subsequently, each sample was placed in the chamber of the micro-CT machine (Skyscan1276, Bruker, Germany) and scanned along the long axis of the specimen at 70 kV and 200 μA for 10 min. The image data of each sample was exported into the CT-Analyser and CTvox (Bruker, Germany) to render the 3D images of the microstructures around the perforation in the tibia. In order to calculate the bone volume density (BV/TV), the region of interest (ROI) was defined as a cylindrical space (φ = 1.8 mm and h = 1.5 mm) growing inwards from the perforation hole. The BV/TV values were calculated based on the grey-value difference between the remnant cement and newly formed bone tissue.
2.7. Statistical analysis
The OriginPro 20 (OriginLab, Massachusetts, U.S.A.) software was used in the statistical analysis of our data. In the comparison of multi-groups (n ⩾ 3), one-way ANOVA was performed to compare values among individual groups (p < 0.05); in the comparison between two groups, unpaired two-sample t-test was performed to investigate the significant level (p < 0.05).
3. Results
3.1. The impacts of LA on CPSC material properties
The effects of LA on the CPSC setting time, compressive strength and porosity are presented in figure 1. In figure 1(a), it is showed that the addition of LA increased both the averaged setting time and compressive strength of CPSC in a concentration-dependent manner. The increases in the setting time and compressive strength suggest that LA might interfere with the hydration process of CPSC. Moreover, the 7 d compressive strengths were higher than those of samples hydrating for 3 d (e.g. in CPSC-15LA, 24.67 MPa vs. 47.46 MPa), implying that the mechanism of LA effects on CPSC hydration was complex and could not be explained without further evidence. The impacts of LA on CPSC porosity are depicted in figure 1(b). Unlike the setting time or compressive strength, the averaged porosity of CPSC was decreased by LA (24.35% for CPSC vs. 5.45% CPSC-15LA); however, the decrease in the porosity for LA-CPSC samples (e.g. CPSC-4LA vs. CPSC-8LA) was much smaller than that between CPSC and LA-CPSC (CPSC vs. CPSC-4LA). These findings strongly indicate that LA might be able to change the microstructure of CPSC and, therefore, affected the CPSC material properties.
Figure 1. (a) The average setting time and compressive strength (3 and 7 d) of CPSC blended with different amount of LA. The columns represent the 3 and 7 d compressive strengths and the hollow circle represents the setting time. The error bar is equal to one standard deviation (n = 4). (b) The changes in the macroporosity of CPSC with different amount of LA after 7 d of hydration. The error bar represents one standard deviation (n = 4).
Download figure:
Standard image High-resolution imageFigure 2 shows the XRD patterns for CPSC and LA-CPSC samples after 3 and 7 d hydrations. The peaks at the 2θ positions of 18° and 34° could be assigned to the formation of portlandite (Ca(OH)2) and the peak at 29° was attributed to remanent C3S (figure 2(a) only). The saddle-like peaks found between 31° and 33° were resulted from the combined diffractions of C2S, C3S and apatite. In addition, it is found that the apatite formation was only detectable in LA-CPSC after 3 d of hydration; however, the apatite peaks could be observed in all samples after 7 d of hydration. Moreover, it is showed that the peak intensities of portlandite significantly decreased in 7 d hydrated samples than the 3 d ones. In figure 2(b), it is also indicated that calcite (CaCO3) was formed in all samples after 7 d of hydration.
Figure 2. The XRD patterns of CPSC and LA-CPSC samplers after (a) 3 d and (b) 7 d hydrations. The reference XRD patterns (PDF cards) were provided in the supplementary file (figure S1 (available online at stacks.iop.org/BMM/16/045042/mmedia)).
Download figure:
Standard image High-resolution imageThe changes in CPSC microstructures by LA were also evaluated by SEM and presented in figure 3. In the SEM images, it is clearly showed that calcium silicate hydrate (C-S-H) gels were formed in all samples but appeared to be bigger with the hydration duration (7 d vs. 3 d). Moreover, apatite crystals were found to precipitate on the surfaces of C-S-H gels after 7 d of hydration, confirming the results showed in the XRD analyses (figure 2).
Figure 3. The SEM images of CPSC samples with different amount of LA after 3 and 7 d of hydration. One scale bar represents 2 µm (n = 3).
Download figure:
Standard image High-resolution image3.2. LA-CPSC in vitro biocompatibility
The biocompatibility of LA-CPSC was first evaluated based on its cytotoxicity on osteoblast cells (figure 4). The relative cell viability was calculated based on the cell viability in the LA-CPSC extract media normalized by the cell viability in the CPSC extract medium (the control). In general, LA-CPSC showed no cytotoxicity to osteoblast cells. In addition, it is found that, in the low LA-concentration groups (CPSC-4LA and CPSC-8LA), the incubation time of LA-CPSC sample in PBS tended to increase the cell viability (7 d vs. 3 d, 115% ± 5.75% vs. 100% ± 5% for CPSC-4LA and 110% ± 5.5% vs. 101% ± 5.05% for CPSC-8LA). Moreover, the extract culture medium prepared by incubating the CPSC-4LA sample in PBS for 7 d showed the highest cell viability and, therefore, this extract culture medium was used in the following genetic analyses.
Figure 4. The normalized cell viability of osteoblast cells incubated with the LA-CPSC extract culture media with respect to the osteoblast cells incubated with CPSC extract culture medium. The extracts were prepared by immersing the CPSC or LA-CPSC samples in PBS for 3 and 7 d. The cell viability was evaluated by incubating the cells with extract culture medium for 1 d. The viability of osteoblast cells incubating with CPSC extract culture medium served as the control group. The normalized cell viability was calculated as viabilityLA-CPSC/viabilitycontrol. The error bar represents one standard deviation (n = 3). * indicates the statistical significance at the level of p < 0.05.
Download figure:
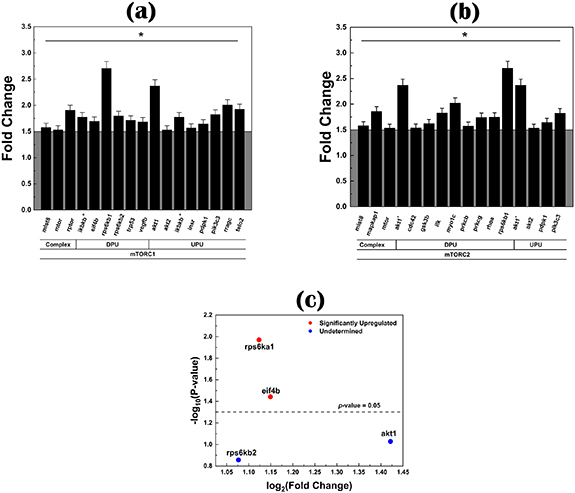
Standard image High-resolution imageThe PCR analyses were performed to investigate the effects of LA-CPSC on the gene expressions via the mTOR signalling pathway. The genes related to the mTOR signalling pathway were first analyzed by the PCR array (84 genes) and further investigated by the real-time PCR (four genes). Because there are two complexes in the mTOR signalling pathway (mTORC1 and mTORC2), the statistically upregulated genes found in mTORC1 and mTORC2 complexes are presented in figures 5(a) and (b), respectively. In the mTORC1 complex, 16 genes, including akt1, akt2, etc, were found to be statistically upregulated (p < 0.05) in the CPSC-4LA group as compared to the CPSC (control) group; while in the mTORC2 complex, there were 15 genes found statistically upregulated by the PCR array. Based on these statistically upregulated genes, four genes, akt1, rps6kb2, eif4b and rps6ka1, were further analyzed by the real-time PCR analysis (figure 5(c)) and it is found that only eif4b and rps6ka1 genes were significantly upregulated (fold change > 2) in the CPSC-4LA group as compared to the control group (p < 0.05).
Figure 5. The fold change of statistically upregulated genes from the (a) mTORC1 and (b) the mTORC2 in osteoblast cells incubated with the CPSC-4LA extract culture medium with respect to the cells incubated with the CPSC (the control group) extract culture medium. Statistically upregulated genes are defined as the fold change greater than 1.5 at p < 0.05 (*). The selected four genes (rps6ka1, eif4b, akt1 and rps6kb2) were further analyzed by the real-time PCR and the fold changes of these four genes are presented in (c). In the PCR array study, there were three biological replicates (n = 3) and, in the real-time PCR analysis, each group contained three technical replicates (n = 3). One error bar represents one standard deviation.
Download figure:
Standard image High-resolution image3.3. LA-CPSC in vivo effects on osteoporotic bone regeneration
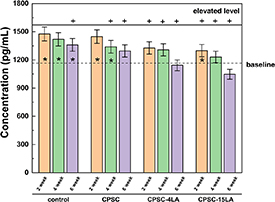
The biomarker study was performed to evaluate the changes of TRACP-5b in serum with respect to the different treatments (i.e. prior to ovariectomy, post-steroid injection and LA-CPSC implantation). The two levels presented in figure 6, the baseline level and the elevated level, were the serum concentrations of TRACP-5b measured in the samples taken before the ovariectomies and after the dexamethasone injections, respectively. It is clearly showed that the treatments of ovariectomy and steroid injection significantly increased the TRACP-5b levels in the serums, implying that the state of OP might be successfully induced by these treatments. The TRACP-5b levels in the serums collected at different endpoints (2, 4 and 6 weeks) are presented by the bar charts in figure 6. After implanted with CPSC-15LA and CPSC-4LA, the OP-induced rats showed significant decreases in the serum levels of TRACP-5b, implying that the osteoclastic activities were significantly reduced after the treatment of LA. By the end of the 6th week, the serum level of TRACP-5b was lower in the CPSC-15LA group than the CPSC-4LA group. In the groups of CPSC and control, the serum TRACP-5b levels also decreased with time but were significantly higher than the CPSC-15LA group by the end of observation. The aforementioned results strongly indicate that LA might be important in suppressing the osteoclastic activities and the effects seemed to be closely dependent on the LA concentration.
Figure 6. The changes in the serum level of TRACP-5b in the osteoporotic rats after 2, 4 and 6 weeks of implantation of LA-CPSC or CPSC. The upper solid line and the lower dash line are referred to as the elevated TRACP-5b level as a result of osteoporosis and the normal serum level of TRACP-5b in healthy rats, respectively. * and + represent that the serum level of TRACP-5b in each group was significantly different from either the baseline or the elevated level (p< 0.05). Each error bar represents one standard deviation (n = 4 biological replicates).
Download figure:
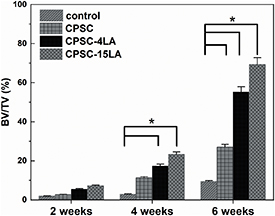
Standard image High-resolution imageThe micro-CT images of bone perorations after 2, 4 and 6 weeks are presented in figure 7 and the BV/TV values of ROI were calculated and depicted in figure 8. By the end of the 2nd week, it is found that the difference in the new bone formation was invisible between CPSC (figure 7(b1-1)), CPSC-4LA (figure 7(c1-1)), CPSC-15LA (figure 7(d1-1)) and the sham group (figure 7(a1)). From the 2nd week to the 4th week, the remanent cement (in red) showed some degradations in the CPSC, CPSC-4LA and CPSC-15LA groups and the new bone formation increased in all groups (figures 7(a2), (b2-1), (c2-1) and (d2-1)). At the end of the entire observation (the 6th week), the CPSC-15LA, CPSC-4LA and CPSC groups demonstrated increased densities of trabecular bones and degradations of remanent cements (figures 7(b3-1), (c3-1) and (d3-1)). In addition, it is appeared that the new bone formation was the highest in the CPSC-15LA group. However, the new bone density was showed to decrease in the control group, implying that the osteoclastic activities might outweigh the capacity of bone regeneration in the control group. In figure 8, the BV/TV values supported the qualitative observations in figure 7. It is showed that the CPSC-15LA group demonstrated the highest bone densities in all four groups at three endpoints and the increase in the bone density was also the largest in the CPSC-15LA group, suggesting that the addition of LA could significantly increase the bone density in a concentration-dependent manner.
Figure 7. The micro-CT images of bone fractures treated by the sham operation (a), CPSC (b), CPSC-4LA (c), CPSC-15LA (d) for 2, 4 and 6 weeks. The sham group served as the control group. The green color represents newly formed trabecular bone and the red color indicates remnant CPSC cement. The scale bar is equal to 1.5 mm.
Download figure:
Standard image High-resolution imageFigure 8. The BV/TV values of bone fractures treated by the control operation, CPSC, CPSC-4LA and CPSC-15LA for 2, 4 and 6 weeks. The BV/TV values are derived from the micro-CT analyses and calculated as the area occupied by newly formed bone divided by the area of ROI (n = 4). * indicates that the difference between two values was statistically significant at the level of p < 0.05. One error bar represents one standard deviation.
Download figure:
Standard image High-resolution image4. Discussion
Although LA has been showed to reduce the osteoclastic activities and have the potential to alleviate OP, its effects on the CPSC material properties remained unknown and were investigated in this present study. It is found that both the setting time and compressive strength of CPSC increased with LA. The CPSC precursor consists of C2S, C3S and MCP and reacts with H2O to become a slurry, which eventually hardens with time. During the hydration (reaction with H2O) process, C3S reacts with H2O at a much fast rate than C2S to form the C-S-H gel and Ca(OH)2; MCP subsequently forms apatite and H2O with Ca(OH)2 [13]. The setting time describes how fast CPSC reacts with H2O and the slurry increases its initial strength. The increase in the setting time strongly suggest that LA can interrupt the hydration of CPSC and delay the initial formation of C-S-H gels. It may be attributed to the adsorption of acetate ions (CH3COO−) onto unreacted C3S, preventing the C3S further dissolution and precipitation.
Although the setting time is indicative of the initial strength of CPSC slurry, the 3 and 7 d compressive strengths in our study show how mechanical stable CPSC can be after a substantial amount of time. This strength can be attributed to both the degree of C-S-H gel polymerization and the level of porosity. As discussed above, the C-S-H particles are formed as a result of both C2S and C3S hydration and polymerize into the long chain of C-S-H gel with time. The formation of C-S-H gel can be interpreted on the XRD patterns. The XRD results reveal no C3S peaks after 7 d of hydration in all samples (figure 2(b)), indicating that C3S has been completely reacted in the 7 d hydrated samples. This result explains that the 7 d compressive strengths of all samples are higher than those of 3 d hydrated samples. It is also showed that apatite was formed in all LA-CPSC samples but not in the CPSC group after 3 d of hydration (figure 2(a)). This may be because negatively-charged acetate ions are highly nucleophilic and result in the solution rich in hydroxide ions (OH−), which can react with MCP to from apatite [19]. The precipitation of apatite crystals fills in the macroscopic voids in C-S-H gel and effectively decreases the CPSC porosity (figure 1(a)). The mechanical strength is inversely related to the porosity and, hence, increases with LA in our study. The SEM results reveal that the apatite particles are much smaller in size and randomly distributed in the C-S-H gel (figure 3).
In the in vitro biocompatibility analyses, the LA-CPSC samples were first evaluated based on their cytotoxicity to osteoblast cells. This cytotoxicity test was performed based on the extract method and osteoblast cells were incubated with the culture medium containing the LA-CPSC extracts (samples immersing in PBS for 3 and 7 d, respectively) for 1 d. The cells incubated with the CPSC-containing culture medium served as the control group. It is found that the LA-CPSC showed no cytotoxic effects to osteoblast cells and, on the contrary, increased the number of viable cells with the LA concentration in extract (3 d vs. 7 d). The significant increase in the number of viable cells (p < 0.05) after co-culture strongly indicates that LA may improve the osteoblast proliferation, with similar effects also observed in another study [20]. As shown in figure 4, the cell viability was the highest in the CPSC-4LA group. Therefore, in the subsequent PCR analyses, the gene expressions were only compared between the cells cultured in this particular group and those in the control one. Unlike other studies focusing on some osteogenic-specific genes, we paid more attention to the gene expressions via the mTOR signalling pathway. The mTOR signalling pathway has been proved to be closely related to the osteoblast proliferation and differentiation and includes two distinctive multi-protein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) [21]. The mTORC1 complex can interact with multiple extracellular signals, such as growth factor, energy status, oxygen, amino acid, and controls intracellular activities, e.g. protein synthesis, autophagy, lipid synthesis, etc [22]. On the other hand, mTORC2 complex is responsible for cytoskeletal organization and controls cell survival, metabolism as well as proliferation. In our study, both the PCR array and conventional real-time PCR methods were used. Eighty-four mTOR-related genes were analyzed by the PCR array and several genes were found to be statistically upregulated, e.g. rps6kb1 and akt1 in mTORC1 (figure 5(a)). Therefore, based on the PCR results, four genes, rps6ka1, rps6kb2, eif4b and akt1 genes, were further analyzed by the real-time PCR (figure 5(c)) and only rps6ka1 and eif4b genes were found significantly upregulated (fold change > 2, p < 0.05). It is known that the activated mTOR can phosphorylate p70 kDa ribosomal protein S6 kinase (p70S6K), which is encoded by rps6kb1 gene [23]. Consequently, the activated p70S6K induces the phosphorylation of eukaryotic initiation factor 4B (encoded by the eif4b gene) and regulates protein translation. In our study, these two genes were significantly upregulated, indicating that LA could increase the osteoblast proliferation via the mTOR signalling pathway [24].
In the in vivo study, we evaluated both the effects of LA on the osteoclastic activities and bone regeneration under the osteoporotic conditions. The state of OP was surgically induced in female rats and further enhanced by the serial injections of steroid. Before and after the OP induction, the blood samples were collected and the serum levels of TRACP-5b were measured. The significant increase of TRACP-5b from the baseline to the elevated level (showed in figure 6) strongly indicated that OP was successfully induced in the studied rats, because this particular biomarker is exclusively produced by osteoclast cells and strongly corelated to the osteoclastic activities [25, 26]. Furthermore, the serum levels were closely monitored over 6 weeks after the LA-CPSC implantation. Although there were small decreases in the control (sham) and CPSC groups, it significantly dropped below the baseline level only in the CPSC-4LA and CPSC-15LA groups, indicating that LA could effectively reduce the osteoclastic activities and might be beneficial to the bone regeneration under the osteoporotic conditions. The imaging analyses (figures 7 and 8) demonstrated that new bone formation was much higher in the LA-CPSC groups than the control group or CPSC group. Similar effects were also observed in our previous study with risedronate as the OP treating agent [14]. This can be explained by the reduced osteoclastic activities. The newly formed bone is very susceptible to the osteoclastic resorption during the bone remodelling process. Without LA, these new bone tissues can be easily resorbed by the osteoclast cells, esp. under the osteoporotic conditions. Meanwhile, the BV/TV results indicated that the amount of new bone was dependent on the LA concentration.
Although our study proves that LA-CPSC may be an effective injectable bone cement formulation to repair bone fractures under the osteoporotic conditions, we failed to corelate the LA release profile to the bone regeneration or osteoclastic activity in this present study. This is because the LA concentration in the blood serum is too low to be detected by the conventional methods, such as high-performance liquid chromatography and colorimetry. Moreover, only the serum TRACP-5b level was analyzed in this study. In the future, critical-size bone fractures will be created in large animals, like sheep, to evaluate the LA-CPSC effects on the bone regeneration under the osteoporotic conditions for a much longer observation period (>3 months).
5. Conclusion
In this study, we formulated an injectable LA-loaded CPSC bone cement to repair bone fractures under the osteoporotic conditions. It is showed that LA increased the setting time and compressive strength in a concentration-dependent manner. The characterizations results demonstrated that LA could interfere with the CPSC hydration process by adsorbing onto unreacted calcium silicate and delaying the initial hydration. Moreover, it is found that LA could increase the formation of apatite and decrease the overall porosity, improving the compressive strength of CPSC. The in vitro analyses indicated that LA-CPSC was biocompatible and able to encourage the proliferation of osteoblast cells via the mTOR signalling pathway. In the animal study, LA-CPSC could significantly reduce the osteoclastic activities in the osteoporotic animals. Furthermore, it is showed that the animals implanted with LA-CPSC demonstrated better bone regeneration in the fracture sites than the animals treated with CPSC alone or sham operation. In conclusion, our results demonstrated that LA-CPSC might be a viable solution to repair bone fractures under the osteoporotic conditions without adverse side-effects.
Acknowledgments
The authors disclosed receipts of the following financial supports for the research, authorship, and publication of this article: This work was sponsored by the Project 51502034 supported by National Natural Science Foundation of China; Project 51872042 supported by National Natural Science Foundation of China; and Project N181908006 supported by Fundamental Research Funds for Northeastern University.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declared no conflict of interest in this article.