Abstract
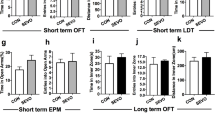
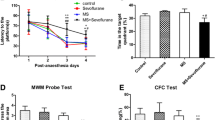
Increasing studies report that prolonged or multiple anaesthetic exposures early in life are associated with detrimental effects on brain function. Although studies have evaluated the detrimental effects on neurocognitive function, few have focused on long-term neuropsychiatric effects. In the present study, C57BL/6 mice received either three neonatal isoflurane exposures or control exposure. Starting on postnatal day 45, the mice were either exposed or not to a chronic variable stress (CVS) paradigm, and CVS-related neuropsychiatric performance was evaluated using a series of behavioural tests. The expression levels of histone 3 lysine 9 acetylation (acetyl-H3K9), brain-derived neurotrophic factor (BDNF), cAMP response element-binding protein-binding protein, and histone deacetylases 1–4 in the amygdala were measured by immunoblotting or immunohistochemistry analysis. In mice with neonatal isoflurane exposure, the effects of sodium butyrate (NaB), a commonly used HDAC inhibitor, were examined on CVS-related behavioural and molecular alterations. The results showed that repeated neonatal isoflurane exposure did not affect innate depression-like and anxiety-like behaviours under non-stress conditions but facilitated the CVS-induced anxiety-like behavioural phenotype. Increased HDAC2 expression in the amygdala was associated with an increase in the CVS-induced repression of acetyl-H3K9 and BDNF expression and an enhanced CVS-evoked anxiety-like behavioural phenotype in mice neonatal isoflurane exposure. NaB significantly decreased the CVS-induced anxiety level by elevating acetyl-H3K9 and BDNF expression. These results suggested that early anaesthesia exposure facilitated chronic stress-induced neuropsychiatric outcomes, and the HDAC2-related epigenetic dysregulation of BDNF gene expression is involved in the underlying mechanism.







Similar content being viewed by others
Abbreviations
- CVS:
-
Chronic Variable Stress
- CNS:
-
Central Nervous System
- OFT:
-
Open-Field Test
- EPM:
-
Elevated Plus Maze Test
- FST:
-
Forced Swim Test
- TST:
-
Tail Suspension Test
- LTP:
-
Long Term Potentiation
- CBP:
-
cAMP Response Element-Binding Protein (CREB)-Binding Protein
- HATs:
-
Histone Acetyltransferases
- HDACs:
-
Histone Deacetylases
- HDACi:
-
Histone Deacetylases Inhibitor
- BDNF:
-
Brain-Derived Neurotrophic Factor
- NaB:
-
Sodium Butyrate
References
Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME, consortium GAS (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387:239–250. https://doi.org/10.1016/S0140-6736(15)00608-X
Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX (2016) Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315:2312–2320. https://doi.org/10.1001/jama.2016.6967
McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, Grobler A, Stargatt R, Hunt RW, Sheppard SJ, Marmor J, Giribaldi G, Bellinger DC, Hartmann PL, Hardy P, Frawley G, Izzo F, von Ungern Sternberg BS, Lynn A, Wilton N, Mueller M, Polaner DM, Absalom AR, Szmuk P, Morton N, Berde C, Soriano S, Davidson AJ, Consortium GAS (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393:664–677. https://doi.org/10.1016/S0140-6736(18)32485-1
Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson AC, Buenvenida SL, Gleich SJ, Wilder RT, Sprung J, Warner DO (2017) Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology 127:227–240. https://doi.org/10.1097/ALN.0000000000001735
Glatz P, Sandin RH, Pedersen NL, Bonamy AK, Eriksson LI, Granath F (2017) Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr 171:e163470. https://doi.org/10.1001/jamapediatrics.2016.3470
Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM (2012) Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology 117:494–503. https://doi.org/10.1097/ALN.0b013e3182644684
Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, Brady JE, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Wood AJ, Li G, Sun LS (2014) Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology 120:1319–1332. https://doi.org/10.1097/ALN.0000000000000248
Raper J, Alvarado MC, Murphy KL, Baxter MG (2015) Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology 123:1084–1092. https://doi.org/10.1097/aln.0000000000000851
Raper J, De Biasio JC, Murphy KL, Alvarado MC, Baxter MG (2018) Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth 120:761–767. https://doi.org/10.1016/j.bja.2018.01.014
Coleman K, Robertson ND, Dissen GA, Neuringer MD, Martin LD, Cuzon Carlson VC, Kroenke C, Fair D, Brambrink AM (2017) Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology 126:74–84. https://doi.org/10.1097/aln.0000000000001383
Ji C, Ni Q, Chen W, Yang Z, Ma D (2019) General anesthetic neurotoxicity in the young: Mechanism and prevention. Neurosci Biobehav Rev 107:883–896. https://doi.org/10.1016/j.neubiorev.2019.10.003
Wu Z, Zhao P (2018) Epigenetic alterations in anesthesia-induced neurotoxicity in the developing brain. Front Physiol 9:1024. https://doi.org/10.3389/fphys.2018.01024
Jia M, Liu WX, Yang JJ, Xu N, Xie ZM, Ju LS, Ji MH, Martynyuk AE, Yang JJ (2016) Role of histone acetylation in long-term neurobehavioral effects of neonatal Exposure to sevoflurane in rats. Neurobiol Dis 91:209–220. https://doi.org/10.1016/j.nbd.2016.03.017
Dalla Massara L, Osuru HP, Oklopcic A, Milanovic D, Joksimovic SM, Caputo V, DiGruccio MR, Ori C, Wang G, Todorovic SM, Jevtovic-Todorovic V (2016) General anesthesia causes epigenetic histone modulation of c-Fos and brain-derived neurotrophic factor, target genes important for neuronal development in the immature rat hippocampus. Anesthesiology 124:1311–1327. https://doi.org/10.1097/ALN.0000000000001111
Zhong T, Guo Q, Zou W, Zhu X, Song Z, Sun B, He X, Yang Y (2015) Neonatal isoflurane exposure induces neurocognitive impairment and abnormal hippocampal histone acetylation in mice. PLoS One 10:e0125815. https://doi.org/10.1371/journal.pone.0125815
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. https://doi.org/10.1038/nature14188
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. https://doi.org/10.1371/journal.pbio.1000412
Watson RE, Desesso JM, Hurtt ME, Cappon GD (2006) Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B Dev Reprod Toxicol 77:471–484. https://doi.org/10.1002/bdrb.20090
Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z (2012) The inhalation anesthetic isoflurane increases levels of proinflammatory TNF-alpha, IL-6, and IL-1beta. Neurobiol Aging 33:1364–1378. https://doi.org/10.1016/j.neurobiolaging.2010.11.002
Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE (2008) The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol 64:618–627. https://doi.org/10.1002/ana.21548
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364. https://doi.org/10.1007/bf00187257
Herman JP, Adams D, Prewitt C (1995) Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61:180–190. https://doi.org/10.1159/000126839
Peng L, Zhu M, Yang Y, Weng Y, Zou W, Zhu X, Guo Q, Zhong T (2019) Neonatal lipopolysaccharide challenge induces long-lasting spatial cognitive impairment and dysregulation of hippocampal histone acetylation in mice. Neuroscience 398:76–87. https://doi.org/10.1016/j.neuroscience.2018.12.001
Preacher KJ, Hayes AF (2004) SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 36:717–731. https://doi.org/10.3758/bf03206553
Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E (2011) Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35:1291–1301. https://doi.org/10.1016/j.neubiorev.2011.02.003
Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J (2019) Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev 99:101–116. https://doi.org/10.1016/j.neubiorev.2018.12.002
Scheich B, Csekő K, Borbély É, Ábrahám I, Csernus V, Gaszner B, Helyes Z (2017) Higher susceptibility of somatostatin 4 receptor gene-deleted mice to chronic stress-induced behavioral and neuroendocrine alterations. Neuroscience 346:320–336. https://doi.org/10.1016/j.neuroscience.2017.01.039
Monteiro S, Roque S, de Sá-Calçada D, Sousa N, Correia-Neves M, Cerqueira JJ (2015) An efficient chronic unpredictable stress protocol to induce stress-related responses in C57BL/6 mice. Front Psychiatry 6:6. https://doi.org/10.3389/fpsyt.2015.00006
Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T (2011) Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology 115:979–991. https://doi.org/10.1097/ALN.0b013e318234228b
Diana P, Joksimovic SM, Faisant A, Jevtovic-Todorovic V (2020) Early exposure to general anesthesia impairs social and emotional development in rats. Mol Neurobiol 57:41–50. https://doi.org/10.1007/s12035-019-01755-x
Autry AE, Monteggia LM (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258. https://doi.org/10.1124/pr.111.005108
Notaras M, van den Buuse M (2020) Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry 25:2251–2274. https://doi.org/10.1038/s41380-019-0639-2
Duman RS, Deyama S, Fogaça MV (2021) Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci 53:126–139. https://doi.org/10.1111/ejn.14630
Miao Z, Mao F, Liang J, Szyf M, Wang Y, Sun ZS (2018) Anxiety-related behaviours associated with microRNA-206-3p and BDNF expression in pregnant female mice following psychological social stress. Mol Neurobiol 55:1097–1111. https://doi.org/10.1007/s12035-016-0378-1
Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 103:13208–13213. https://doi.org/10.1073/pnas.0605180103
Boyle LM (2013) A neuroplasticity hypothesis of chronic stress in the basolateral amygdala. Yale J Biol Med 86:117–125
Wu J, Bie B, Naguib M (2016) Epigenetic manipulation of brain-derived neurotrophic factor improves memory deficiency induced by neonatal anesthesia in rats. Anesthesiology 124:624–640. https://doi.org/10.1097/aln.0000000000000981
Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V (2006) General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis 11:1603–1615. https://doi.org/10.1007/s10495-006-8762-3
Tang XL, Zhao YL, Zhou ZQ, Yan J, Zhou BY, Chi XH, Luo AL, Li SY (2020) Resveratrol mitigates sevoflurane-induced neurotoxicity by the SIRT1-dependent regulation of BDNF expression in developing mice. Oxid Med Cell Longev 2020:9018624. https://doi.org/10.1155/2020/9018624
Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10:423–433. https://doi.org/10.1038/nrn2651
Sharp BM (2017) Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry 7:e1194. https://doi.org/10.1038/tp.2017.161
Lomazzo E, König F, Abassi L, Jelinek R, Lutz B (2017) Chronic stress leads to epigenetic dysregulation in the neuropeptide-Y and cannabinoid CB1 receptor genes in the mouse cingulate cortex. Neuropharmacology 113:301–313. https://doi.org/10.1016/j.neuropharm.2016.10.008
Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC (2013) Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry 73:763–773. https://doi.org/10.1016/j.biopsych.2013.01.012
Singh P, Thakur MK (2018) Histone deacetylase 2 inhibition attenuates downregulation of hippocampal plasticity gene expression during aging. Mol Neurobiol 55:2432–2442. https://doi.org/10.1007/s12035-017-0490-x
Davie JR (2003) Inhibition of histone deacetylase activity by butyrate. J Nutr 133:2485s–2493s. https://doi.org/10.1093/jn/133.7.2485S
Citraro R, Leo A, De Caro C, Nesci V, Cantafio MGE, Amodio N, Raso GM, Lama A, Russo R, Calignano A, Tallarico M, Russo E, De Sarro G (2020) Effects of histone deacetylase inhibitors on the development of epilepsy and psychiatric comorbidity in WAG/Rij rats. Mol Neurobiol 57:408–421. https://doi.org/10.1007/s12035-019-01712-8
Han A, Sung YB, Chung SY, Kwon MS (2014) Possible additional antidepressant-like mechanism of sodium butyrate: targeting the hippocampus. Neuropharmacology 81:292–302. https://doi.org/10.1016/j.neuropharm.2014.02.017
Singh P, Konar A, Kumar A, Srivas S, Thakur MK (2015) Hippocampal chromatin-modifying enzymes are pivotal for scopolamine-induced synaptic plasticity gene expression changes and memory impairment. J Neurochem 134:642–651. https://doi.org/10.1111/jnc.13171
Hayase T (2016) Putative epigenetic involvement of the endocannabinoid system in anxiety- and depression-related behaviors caused by nicotine as a stressor. PLoS One 11:e0158950. https://doi.org/10.1371/journal.pone.0158950
Schroeder FA, Lin CL, Crusio WE, Akbarian S (2007) Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 62:55–64. https://doi.org/10.1016/j.biopsych.2006.06.036
Fernando W, Martins IJ, Morici M, Bharadwaj P, Rainey-Smith SR, Lim WLF, Martins RN (2020) Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. J Alzheimers Dis 74:91–99. https://doi.org/10.3233/jad-190120
Rane P, Shields J, Heffernan M, Guo Y, Akbarian S, King JA (2012) The histone deacetylase inhibitor, sodium butyrate, alleviates cognitive deficits in pre-motor stage PD. Neuropharmacology 62:2409–2412. https://doi.org/10.1016/j.neuropharm.2012.01.026
Zuzina AB, Vinarskaya AK, Balaban PM (2020) Histone deacetylase inhibitors rescue the impaired memory in terrestrial snails. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 206:639–649. https://doi.org/10.1007/s00359-020-01422-w
Vinarskaya AK, Balaban PM, Roshchin MV, Zuzina AB (2021) Sodium butyrate as a selective cognitive enhancer for weak or impaired memory. Neurobiol Learn Mem 180:107414. https://doi.org/10.1016/j.nlm.2021.107414
Wilson CB, McLaughlin LD, Ebenezer PJ, Nair AR, Francis J (2014) Valproic acid effects in the hippocampus and prefrontal cortex in an animal model of post-traumatic stress disorder. Behav Brain Res 268:72–80. https://doi.org/10.1016/j.bbr.2014.03.029
Sivaprakasam S, Prasad PD, Singh N (2016) Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther 164:144–151. https://doi.org/10.1016/j.pharmthera.2016.04.007
Yang LL, Millischer V, Rodin S, MacFabe DF, Villaescusa JC, Lavebratt C (2020) Enteric short-chain fatty acids promote proliferation of human neural progenitor cells. J Neurochem 154:635–646. https://doi.org/10.1111/jnc.14928
Funding
This research was supported by the Natural Science Foundation of Hunan Province, RP China [2018JJ2627].
Author information
Authors and Affiliations
Contributions
Study design: LP, QG, and TZ. Data collection and analysis: LP and XL. Reagents/materials/analysis tools: XL and YY. Wrote the manuscript: LP and TZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, L., Liu, X., Yang, Y. et al. Histone Deacetylase 2-Mediated Epigenetic Regulation is Involved in the Early Isoflurane Exposure-Related Increase in Susceptibility to Anxiety-Like Behaviour Evoked by Chronic Variable Stress in Mice. Neurochem Res 46, 2333–2347 (2021). https://doi.org/10.1007/s11064-021-03368-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03368-0