Abstract
Soil, a connecting link between biotic and abiotic components of terrestrial ecosystem, receives different kinds of pollutants through various point and nonpoint sources. Among different sources of soil pollution, contaminated irrigation water is one of the most prominent sources affecting soils throughout the globe. The irrigation water (both surface and groundwater) is increasingly getting polluted with contaminants such as metal(loid)s due to various anthropogenic activities. The present study was conducted to analyze metal(loid) contents in agricultural soil samples (N = 24) collected from fields along the banks of rivers Beas and Sutlej flowing through Punjab state of India, using wavelength-dispersive X-ray fluorescence (WDXRF) spectroscopy. The soil samples were also analyzed for their genotoxic potential using Allium cepa root chromosomal aberration assay. The rivers Beas and Sutlej are contaminated with municipal and industrial effluents in different parts of Punjab. The soil samples analyzed were found to have higher contents of arsenic, cobalt and chromium in comparison with the reference values given by various international agencies. Pollution assessment using different indices like index of geo-accumulation, enrichment factor and contamination factor revealed that the soil samples were highly polluted with cobalt and arsenic. The Allium cepa assay revealed that maximum genotoxicity was found in soil samples having higher contents of As and Co. Pearson’s correlation analysis revealed strong positive correlation between the different metal(loid)s which indicated common sources of these metal(loid)s. Therefore, efforts must be taken to reduce the levels of these metal(loid)s in these agricultural soils.
Similar content being viewed by others
A polluted aquatic ecosystem is a critical threat to the environment as it can have an immediate effect on the sustenance of life. Most severe sources of pollution are related to the disposal of untreated and partially treated industrial wastes that contain hazardous toxic substances (such as metal(loid)s) most of which are not completely degradable and potentially harmful. Due to continued disposal of industrial wastes, many hydrological ecosystems in developing countries are stressed beyond repair (Singh et al. 2017; Rigo et al. 2020).
Soil can concentrate contaminants it receives from different sources, and they (contaminants) move down stream and accumulate in catchment area, sediments and biological tissues (Juarez-Santacruz et al. 2013; Bhatti et al. 2018). Among various contaminants identified, the presence of potentially toxic metal(loid)s is a major concern because these are water soluble and can easily enter the food chains. They have been reported to combine with proteins, nucleic acids and other biomolecules, thus impairing their functions (Gastaldo et al. 2007; Sharma et al. 2019).
On a global basis, agriculture is the single largest user of fresh water and because of the presence of various metal(loid)s and other toxic chemicals in irrigation water, it contributes significantly to the accumulation of metal(loid)s in soil. Excessive accumulation of metal(loid)s in agricultural soils not only results in soil contamination, but also affects food quality and human health (Zhang et al. 2019; Bhatti et al. 2020). From contaminated soils, metal(loid)s can leach to lower layers of soil and ultimately contaminate the groundwater beyond certain concentrations. Also, runoff from the polluted agricultural fields can lead to contamination of the nearby surface water bodies (Cobela-Garcia and Prego 2003; Chandrasekaran et al. 2015; Sharma et al. 2019). Because of their non-biodegradable nature, long biological half-lives and potential to accumulate in different body parts, metal(loid)s are harmful for human beings (Mudgal et al. 2010). Generally, plants require some metal(loid)s in trace amounts, but their presence in large quantities severely affects physiology and biochemical functions (Nagajyoti et al. 2010). Some of the heavy metals like Pb, Cd, Cr, and Hg are non-essential for plants and can adversely affect plant growth even at lower concentration (Khan et al. 2015).
Researchers around the globe are using different analytical methods to evaluate the elemental composition of environmental samples (Bielecka et al. 2014; Soodan et al. 2014a). X-ray fluorescence (XRF) is one of the analytical methods, which is fast, accurate, non-destructive and is used to determine the chemical composition of solids and liquids (Yusuf et al. 2014). There are two main variants, energy (EDXRF) and wavelength-dispersive XRF spectrometer (WDXRF) systems, with the ability to detect a wide range of elements. EDXRF goes from sodium to uranium, while WDXRF can detect metals ranging from beryllium to uranium. These can analyze concentrations as low as sub-ppm to 100% (Funtua 1996).
Hazardous and risk assessment of soil samples is usually carried out by analysis of various physico-chemical parameters. However, these parameters are not sufficient for biological risk assessment because physico-chemical analysis alone is not able to provide information on effect of chemical compounds and does not consider the interaction between contaminants, soil matrix and biota (Bhatti et al. 2017; Hu et al. 2019). Therefore, the use of bioassays is equally important to estimate ecological risk in soil or other matrices. Among different bioassays used, plant bioassays are widely used for initial screening methods to determine the genotoxic potential of chemicals, drugs or pollutants. Allium cepa root chromosomal aberration assay is one of the plant bioassays which has been validated by International Programme on Chemical Safety (IPCS) under auspices of World Health Organization (WHO) and United Nations Environment Programme (UNEP) for determination of genotoxicity of various agent including soil samples (Cabrera and Rodriguez 1999; Hammann et al. 2020).
Punjab is a north-western state of India located in Indo-Gangetic alluvial plains extending from 29°30ʹ N to 32°32ʹ N latitude and 73°52ʹ E to 76°56ʹ E longitude. Beas and Sutlej are important tributaries of Indus River system and traverse most of the part of Punjab plains. The water of these two rivers is used for irrigation in Punjab. Both these rivers culminate at Harike wetland, a Ramsar site in district Tarn-Taran of Punjab plains. After their culmination at this Ramsar site, the joint stream of Beas and Sutlej rivers is known as Sutlej. During their flow in Punjab and even before entering Punjab, these rivers receive industrial and municipal wastes of various districts such as Kangra, Pathankot, Amritsar, Jalandhar and Ludhiana. Major industries in these districts are related to pharmaceuticals, paper, chemical, textile and sugar manufacturing and thermal power plants. In India there are several laws that regulate the disposal of industrial and domestic waste water such as Water (Prevention and Control of Pollution) Cess Act, 1971; Water (Prevention and Control of Pollution) Act, 1974, which was further amended in 1988; and Environment Protection Act, 1986. But, many times these laws are violated and industrial establishments discharge their untreated wastes directly or indirectly into these rivers which contaminate their water. The increasing pollution in these rivers also poses significant risk of contamination of agricultural soils of Punjab with various contaminants including metal(loid)s, and the food grown on these agricultural fields did not checked for various contaminants including metal(loid)s. Thus, the present study was conducted to assess the contents of metal(loid)s in agricultural soils of six villages situated on the banks of rivers Beas and Sutlej, and pollution status using different indices like Index of geoaccumulation (Igeo), enrichment factor (EF) and contamination factor (Cf). The soil samples were also analyzed for their genotoxic potential using Allium cepa root chromosomal aberration assay.
Materials and Methods
Study area and sample collection
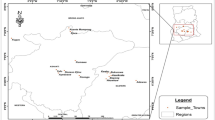
The study area consisted of six villages situated on the banks of rivers Beas and Sutlej in Punjab state of India. Among six villages, four villages are situated on banks of river Beas (upstream to Harike wetland) and two were situated on banks of river Sutlej (downstream to Harike wetland). The soil of the study area is composed of sediments of Shiwalik and Himalayan hills brought down and laid by the rivers Beas and Sutlej. The water of these two rivers is used for irrigation in the study area. Six villages on banks of rivers Beas and Sutlej selected for the present study, Fig. 1 shows the sampling villages along with sample codes and geographical coordinates of soil samples. Soil samples were collected in the month of October, 2012. From each village, four agrarian fields (two under rice and two under sugarcane cultivation) were selected for sampling. Twenty-four composite surface soil samples were collected from horizon ‘A’ of the soil (i.e., 5–15 cm depth). Horizon ‘A’ was particularly chosen because it has been reported to be heavily contaminated with heavy metals and roots of these crops do not penetrate beyond this horizon (Gowd et al. 2010). Samples were collected by using a plastic spatula and transferred to a clean polythene bag to avoid all possible adulterations.
Elemental analysis
Elemental analysis of soil samples was performed using wavelength-dispersive X-ray fluorescence spectroscopy (WDXRF) using a S8 Tiger instrument manufactured by Bruker (Billerica, MA, USA) at Sophisticated Analytical Instrumentation Facility, Panjab University, Chandigarh. The soil samples were air-dried by placing soil on steel mesh containing blotting paper on it. Each air-dried soil sample (9 gm) was ground along with binder tablets (9 tablets/gm of soil sample) to make homogenized fine powder of having particle size less than 50 µm (Lu et al. 2010). Pellets having diameter of 34 mm and thickness 4 mm were made from the ground soil samples by applying a pressure of 15 tons using a hydraulic press. These pellets were then subjected to X-rays for a 30 min run time. Geo-quant, a geological sample dedicated software, was used for sample analysis. To authenticate the quality of chemical analysis data and examine the accuracy of the data, standard soil reference material (GBW07406) was analyzed.
Pollution assessment
Pollution assessment of different soil samples was carried out on the basis of results of elemental analysis and calculating different indices like index of geoaccumulation (Igeo), enrichment factor (EF) and contamination factor (Cf).
Index of geoaccumulation
The index of geoaccumulation (Igeo) compares the content of any element studied in soil sample to its pre-industrial concentration to enable the assessment of contamination of that particular soil (Muller 1969). The index of geoaccumulation can be calculated by using equation
where Cn is the content of element in the soil sample studied, Bn is the concentration of element in the earth’s crust (Taylor and Mclennan 1995), and 1.5 is the constant to allow natural fluctuations in the contents of elements studied. Soils were classified into different classes on the basis of index of geoaccumulation (Igeo) as per Supplementary Table 1.
Enrichment factor
The enrichment factor enables us to assess the enrichment or depletion of element of interest in soil sample by normalizing the content of element studied with a reference element. A reference element must be relatively inert with respect to chemical weathering and which has no significant anthropogenic source and shows low occurrence and variability (Sutherland 2000). Zirconium (Zr) is the element which has been widely used for assessment of depletion of reactive heavy metals in geological samples because of its high chemical stability during weathering processes (Cobela-Garcia and Prego 2003). In nature zirconium exists in oxide or silicate forms and belongs to lithophile elements which accumulate in sediments and have very little circulation in geochemical cycles (Wang et al. 2013). Keeping these in mind, the crustal zirconium (Zr) value of 190 mg/kg (Taylor and Maclennan 1995) was used as reference element. The value of enrichment factor was calculated by using modified formula given by Loska et al. (2004):
where Cn (sample) is content of an element in soil sample, Cref (sample) is the content of the reference element (Zr) in soil sample, Bn (background content) of element in the earth crust (Taylor and Mclennan 1995) and Bref (background) is the content of the reference element (Zr) in the earth crust (Taylor and Mclennan 1995). Soils were classified into different classes on the basis of enrichment factor (EF) as per Supplementary Table 2.
Contamination factor
The contamination factor, as suggested by Hakanson (1980), provides an assessment of contamination level of a soil sample with particular element as compared to its reference value in preindustrial level and is calculated as:
where \(C_{0 - 1}^{i}\) is the average content of a particular element from at least five sampling sites, and \(C_{n}^{i}\) is the content of particular metal in the earth’s crust. Soils were classified into different classes on the basis of contamination factor (Cf) as per Supplementary Table 3.
Estimation of genotoxic potential
Allium cepa root chromosomal aberration assay was performed to evaluate genotoxic potential of collected soil samples. In situ conditions were simulated by setting up pot cultures. For this assay, healthy uniform sized onions were bought from local market. The onion bulbs were allowed to root directly in soil samples contained in small pots. The acid washed sand was used as negative control. After emergence of roots, the onion root tips were washed thoroughly with water; root tips measuring 0.5–1 cm were cut and fixed in Farmer’s fluid (3:1::Ethanol:Glacial Acetic Acid) and kept till further use (15–30 days). The fixed root tips were hydrolyzed in 1 N HCl at 60 °C for 1 min and transferred to a watch glass that contained a mixture of aceto-orcein and 1 N HCl in the ratio of 9:1 for half an hour. The root tips were warmed at regular intervals of 5 min while in the stain. The root tips were then carefully transferred to clean glass slide and squashed in 45% glacial acetic acid under a cover slip. The slides were screened for different types of chromosomal aberrations. The whole experiment was set up in triplicate. From each set up 300, and a total of 900 dividing cells were scored for each sample. Percent aberrant cells were calculated as:
Statistical analysis
The descriptive and other statistics of metal(loid) concentrations in the agricultural soil samples was done using IBM SPSS (Statistical Package for the Social Sciences) software version 16.0 (New York, USA) and PAST (Paleontological Statistics) software version 3.06 (Hammer et al. 2001). Pearson’s correlation analysis was done to determine the correlation between metal(loid)s and genotoxicity parameter of the soil samples.
Results and Discussion
Table 1 summarizes the contents of various metal(loid)s in agricultural soil samples collected from agricultural fields on the banks of river Beas and Sutlej. The results observed for genotoxicity assessment of the soil samples are represented in Tables 2 and 3 which represents the Pearson’s correlation analysis of potentially toxic metal(loid)s and genotoxicity parameters. On the basis of results of elemental analysis, different indices, viz. Igeo, EF and CF, the soil samples, were classified into different categories of contamination and the results were represented as box plots in Figs. 2, 3 and 4, respectively.
Concentrations of metal(loids) in soils
Among the metal(loid)s analyzed in agricultural soil samples in the present study, the contents of arsenic, barium, cobalt and chromium were found to be above maximum permissible limits (CNEMC 1990; C.So.Q.Gs. 2007). The contents of other metal(loid)s, i.e., copper, nickel, lead, strontium, vanadium, zinc, were found to be lower than national and international permissible limits (Table 1). Permissible limits for rubidium, yttrium and zirconium could not be found in the available literature published so far.
The concentrations of arsenic in all the soil samples were found to be higher than the reference value given by Canadian soil quality guidelines (C.So.Q.Gs. 2007). The content of arsenic ranged from 23 to 33 mg/kg with an average value of 27.33 mg/kg. Arsenic is one of the most common contaminants found in soil and waters which finds its entry into the ecosystem from various natural and anthropogenic sources (Goldhaber et al. 2003). The natural sources include various metallic sulfide ores, coal deposits, tectonic movements, etc., while the anthropogenic sources include paints, tanning agents of leather, metal alloys, and wood preservative agents (Welch et al. 2000). Despite the use of arsenic in variety of industrial establishments, its release into the environment occurs more by natural processes (> 60%) than the anthropogenic sources (> 40%) (Ayres and Ayres 1999). The levels of barium in the present study ranged from 399 to 558 mg/kg, having an arithmetic mean of 478.6 mg/kg. In the present study, content of Ba in all the soil samples exceeds the reference value (C.So.Q.Gs. 2007) of 300 mg/kg. In addition to the natural lithogenic sources, the anthropogenic sources of barium include different industries such as soaps, explosives, fire extinguishers, drilling fluids, insecticides. (Ippolito 2006; Menzie et al. 2008). Mobilization of barium in soil is governed by different physico-chemical characteristics of soil. Cobalt content in all the soil samples was found to be more than the reference value of 10.6 mg/kg given by CNEMC (1990). The high levels of Co in soil samples can be attributed to contamination by industrial activities such as alloys, superalloys, chemicals and ceramic production, cemented carbides and steels in nearby areas (Ma and Hooda 2010). According to Stiborova et al. (1988), application of cobalt at lower concentrations improved the root system by helping the plant to absorb water and ultimately the uptake of several nutrients dissolved in this water resulting in better growth of plant. The content of chromium ranged from 78 to 147 mg/kg with an average content of 95.42 mg/kg. According to Wedepohl (1995), the safe value for Cr is 100 mg/kg. Twenty-nine percent of soil samples in the present study exceeded this normal value. Chromium is mainly added to the ecosystem through various anthropogenic activities as it is used in the manufacturing of steel and other alloys, chrome plating and pigment production. Chromium exists in eight oxidation states ranging from − 2 to + 6. Cr+3 and Cr+6 are common occurring states and Cr+6 is most toxic to biological systems. Adsorption of Cr+6 decreases with increase in pH, while that of Cr+3 increases with increase in pH of soil sample (Machender et al. 2011). It has been reported that in most of the industrial effluents, Cr is present in less toxic state (Cr+3) but because of varying environmental conditions Cr+3 is oxidized to toxic state (Cr+6) (Gowd and Govil 2008). The average content of lead in different agricultural soil samples was 22.63 mg/kg and ranged from 19 to 27 mg/kg. A baseline value of 25 mg/kg was estimated for surface soil on global scale, and level above this limit indicates the anthropogenic influence (Gowd et al. 2010). In the present study, only two samples showed values slightly higher than this baseline value. Lead (Pb) is a naturally occurring metal, which occurs ubiquitously in both organic (tetraethyl lead) and inorganic (lead acetate, lead chloride etc.) forms in the environment (Shalan et al. 2005; Jabeen et al. 2010). It is one of the major toxicants in the environment because of its potential hazards to living organisms and is being used by many industries including mining and refining (Marques et al. 2006; Ahmad et al. 2014). Intoxication by lead (Pb) induces a broad range of physiological, biochemical, genetic and behavioral changes in living organisms especially animals which include disorders of nervous system, cardiovascular system, excretory system and reproductive system (Mudgal et al. 2010).
It is to be noted that earlier soil monitoring studies done from the region have analyzed the contents of those metal(loid)s which are either highly toxic (such as As, Cr, and Pb) or those which are important for industrial or agricultural use (such as Cu, Co, Ni, and Zn). But the contents of other rare earth metals (such as Rb, Sr, and V) are seldom analyzed or discussed in monitoring studies from the area of study. So, contents of these metals were analyzed in order to provide preliminary data about their concentrations in soils of the study area. The average content of rubidium in different soil samples was found to be 114.60 mg/kg with a minimum and maximum values of 100 and 135 mg/kg, respectively. Rubidium (Rb) is located between potassium (K) and cesium (Cs) in the periodic table and is a rarely studied abundant alkali metal. The mean content of strontium in different soil samples was found to be 89.58 mg/kg and ranged from 67 to 162 mg/kg. The mobility of strontium is very swift during weathering, particularly in oxidizing acidic environment. Strontium is indicative of calcareous rocks, because of its strong association with calcium (Gowd et al. 2010). In nature, strontium occurs in four stable isotopes (84Sr, 86Sr, 87Sr, and 88Sr). Three of these (84Sr, 86Sr, and 88Sr) are non-radiogenic, while 87Sr is radiogenic and is formed by the radioactive decay of 87Rb, with a half-life of approximately 4.88 × 1010 years (Faure and Mensing 2005). Many scientists around the globe opined that because of large atomic mass, strontium contents change a little while passing through weathered rock to soil and finally to food chain (Ruggeberg et al. 2008). In the present study, content of vanadium (V) ranged from 51 to 90 mg/kg with an average of 68.33 mg/kg. Normal threshold value for vanadium in soil is 100 mg/kg (Larocque and Rasmussen 1998). Vanadium finds its entry into the environment through leaching of rocks, combustion of coal or petroleum products, contamination from the use of phosphate fertilizers (Vachirapatama et al. 2002). Yttrium is categorized as a rare earth element (REE) that occurs in moderate amounts in the environment, i.e. approximately equal in amounts to those of Cr, Co and Zn (Zhang et al. 2015). The content of yttrium ranged from 21 to 32 mg/kg in the present study. According to Wedepohl (1995), the earth’s crust contains 24 mg/kg of yttrium, which is about twice more than lead. Its biological significance has not yet been clearly established, but there are reports available showing disruptive and protective effects of Y on plants and blue green algae, respectively (Maksimović et al. 2014). Zirconium (Zr), popularly known as the gemstone zircon, is a greyish-white metal having atomic number 40, density of 6.506 g/cm3, with five stable isotopes and three unstable isotopes (Allin 2010). It is quite abundant in the Earth’s crust with total percent amounts greater than the combined total of all available copper, nickel, lead, tin, zinc and mercury deposits, making it the 12th most common element in nature (Shahid et al. 2013). Its concentrations in the present study ranged from 147–241 mg/kg (Table 1).
The metal(loid)s analyzed in the present study originated from both natural and anthropogenic sources. Natural sources included parent rock materials and anthropogenic sources included industries, liquid manure, composted manure and agrochemicals including fertilizers and pesticides etc. (Gowd et al. 2010). The increased level of various metal(loid)s is a cause of concern because some of the earlier reports from our lab have pointed out the increased metal(loid)s concentration in the food and fodder crops (Bhatti et al. 2018, 2020), and there are great chances of uptake of metal(loid)s in crops such as wheat, rice and sugarcane growing on the fields of present study area. This can instigate serious concern as the food crops growing in these fields are harvested and taken to the markets established by the state government to be procured by the local consumers directly or by state and national agencies without checking the level of contamination. The high level of metal(loid)s observed can leach to lower layers of soil and ultimately pollute the groundwater of the area. Also, runoff from these polluted agricultural fields can lead to contamination of the nearby surface water bodies (Cobela-Garcia and Prego 2003; Chandrasekaran et al. 2015; Sharma et al. 2019).
In the present study, positive skewness values were observed for all metal(loid)s (except Ni) which indicated that these metal(loid)s skewed toward lower concentrations. In case of kurtosis, positive values were observed for As, Cr, Pb, Rb, Sr and Zn which indicated steeper distribution than normal for these elements, while negative kurtosis values were observed for Ba, Co, Cu, Ni, V, Y and Zr indicating that data for these elements has lighter tails and a flatter peak than normal distribution (Ma et al. 2016).
Metal(loid) contamination levels in soils
In order to assess the level of contamination caused by the metal(loid)s in agricultural soil samples in present study, three indices/factors were analyzed, i.e. index of geoaccumulation (Igeo), enrichment factor (EF) and contamination factor (CF), which are presented in Figs. 2, 3 and 4, respectively. The Igeo values obtained for Co, Cr and Ni indicated that these metals fall in Class 1 (Igeo: 0–1) of this index, which meant that soils samples were unpolluted to moderately polluted with these three metals. The Igeo values observed for As (Class 2) show that soil samples were moderately polluted with As (Igeo: 1–2). The Igeo values for other metal(loid)s were below 0 (Class 0) which meant that soil samples were practically unpolluted with these metal(loid)s. The values obtained in case of enrichment factor (Fig. 3) indicated that soil samples had significant enrichment (Class 3; EF > 5–20) of As and Co, while EF values observed for Cr indicated moderate enrichment of Cr (Class 2; EF > 2–5) in analyzed soils. The EF values observed for other metal(loid)s fell into Class 1 (EF < 2) which pointed towards minimal enrichment of soil samples with these metal(loid)s. The contamination factor (CF) values obtained in the present study showed that Ba, Cu, Sr and Zn caused very low contamination (Cf < 1); Cr, Ni, Pb, Rb, V, Y and Zr caused moderate contamination (1 ≤ Cf < 3), while As and Co caused very high contamination (6 ≤ Cf) in the soil samples analyzed.
Overall, it can be stated that based upon the values observed for Igeo, EF and CF, the soil samples were significantly polluted with As, Co and Cr which indicated towards anthropogenic influences such as industrialization, urbanization and extensive agrochemical-based agriculture (Bhatti et al. 2018; Sharma et al. 2019).
Genotoxic potential
The meristematic cells of Allium cepa roots were analyzed to record the mitotic anomalies induced in root tip cells following in-situ treatment with different soil samples collected from the fields on the bank of rivers Beas and Sutlej, Punjab. Different types of chromosomal aberrations induced in root tip cells Allium cepa were differentiated into physiological and clastogenic aberrations which arise because of abnormalities in spindle fibers and direct action on chromosome, respectively. The spectrum of physiological aberrations includes C-mitosis, delayed anaphase/s, stickiness, laggard/s, vagrant/s, abnormal anaphase/s and abnormal metaphase/s while that of clastogenic aberrations includes chromatin bridge/s, chromosomal break/s and ring chromosome/s. Figure 5 shows different kinds of chromosomal aberrations induced in Allium cepa and suggests the presence of certain cytotoxic/genotoxic substances present in the soil samples. Total chromosomal aberration frequency including both physiological and clastogenic aberrations for different samples ranged from 10.55% to 16% (Table 2). All the soil samples except V1, B1 and B2 were found to have significant genotoxic potential as compared to control. The level of significance was checked at p ≤ 0.05 and 0.01 level indicated by * and **. From the spectrum of physiological and clastogenic aberrations, the most abundant were delayed anaphases and chromatin bridges, respectively. Delayed anaphase is characterized by two anaphasic groups of chromosomes lying close to each other near equatorial plate (Soodan, et al. 2014b), whereas chromatin bridges are formed due to unequal exchanges of chromatin material which results in the formation of dicentric chromosome and subsequently pulled equally to both poles at anaphase (Sax and Sax 1968). In case of most soil samples (except B4 and K4), frequency of cells with delayed anaphases was highest, followed by c-mitosis. C-mitosis is a term coined by Levan (1938) to describe the effects of various physical and chemical agents which act in a fashion similar to that of colchicine. After delayed anaphase and c-mitosis, cells with stickiness were frequent among various physiological aberrations. Kong and Ma (1999) gave a hypothesis that stickiness can be caused because of incomplete separation of daughter chromosomes which were cross-linked by chromoproteins. Sample K1 showed maximum (16%) percentage of total chromosomal aberrations, in the same sample cobalt is 10 times higher than the reference value given by CNEMC (1990). Sample K1 was followed by N4 (15.22%) and B3 and B4 (both 15.11%), respectively, which also have higher content of cobalt as compared to reference value indicating contamination of soil with cobalt because of continuous usage of fertilizers containing cobalt and may be a potential cause of genotoxicity in these soil samples. Results of the present study are in consistence with some previous studies around the globe which evaluated the genotoxic potential of agricultural soil samples (Souza et al. 2013; Soodan et al. 2014b; Bhatti et al. 2018).
The correlation analysis indicated statistically significant correlation among different metal(loid)s (As, Co, Ba, Cu, Ni, Pb, Rb Sr, V, Y, Zn and Zr). The statistically significant positive correlation between most metal(loid)s points towards the common sources of these metal(loid)s in the studied agricultural soils. These include both natural and anthropogenic sources (Chandrasekaran et al. 2015; Bhatti et al. 2018). For example, metal(loid)s such as As, Cr, Cu, Pb, and Zn are found in trace amounts in different agrochemicals such as fertilizers, insecticides, and pesticides (Hu et al. 2019). Industrial effluents from upstream cities such as Ludhiana, Jalandhar and Phagwara are discharged in rivers Sutlej and Beas. The water from these rivers is used as irrigation source for agriculture in the study area which acts as common source for metal(loid)s such as As, Cr, Co, Cu, and Ni. (Bhatti et al. 2020). Also, these metal(loid)s are present in parent rock material worldwide, which is a primary common source of these metal(loid)s in soils (Larocque and Rasmuseen 1998). The higher values of correlation coefficients among different metal(loid)s indicate towards common sources of metal(loid)s, similar pathways of metal(loid) absorption from soils and interdependency of different metal(loid)s for different metabolic processes in soil systems (Bhatti et al. 2020). Overall, it was observed that agricultural soils analyzed in the present study were contaminated with metal(loid)s due to different anthropogenic activities and posed significant genotoxic risks. Therefore, immediate steps must be taken to reduce the levels of metal(loid)s in the soils in order to ensure healthy agricultural practices.
Conclusions
The present study was conducted to analyze the contents of different metal(loid)s in agricultural soil samples collected from six villages situated on the banks of rivers Beas and Sutlej in Punjab, India. The soils were under rice and sugarcane cultivation. Among the metal(loid)s analyzed, contents of As, Co and Cr were found to be above the maximum permissible limits. The factors/indices (index of geoaccumulation, enrichment factor and contamination factor), calculated to determine the metal(loid) contamination, indicated that the studied soils were highly contaminated with metal(loid)s (especially As and Co) which could be attributed to different anthropogenic sources. Allium cepa root chromosomal aberration assay revealed that the soils samples may pose significant genotoxic risks to the exposed living beings, which include organisms such as soil microflora, plants, soil organisms and human beings in contact with the soils. The high contents of toxic metal(loid)s such as As and Cr observed in the present study can lead to genotoxic and mutagenic effects in plants growing in these soils which can further affect the living beings dependent on these plants. Statistically significant positive correlation was observed among the different metal(loid)s in the soil samples which points towards the common sources (natural and anthropogenic) of these metal(loid)s. Overall it was observed that the agricultural soils in the study area were contaminated with metal(loid)s and steps must be taken to reduce their levels.
References
Ahmad A, Toor RH, Aftab S, Shakoori AR (2014) Anti-proliferative and genotoxic effect of Arsenic and Lead on human brain cancer cell line. Pakistan J Zool 46(4):1069–1076
Allin CW (ed) (2010) Encyclopedia of global resources: South Korea-Zirconium; Appendixes; Indexes, vol 4. Salem Press Inc
Awashthi SK (2000) Prevention of Food Adulteration Act no 37 of 1954. Central and State Rules as Amended for 1999. Ashoka Law House, New Delhi
Ayres RU, Ayres L (1999) Accounting for resources, 2: the life cycle of materials. Edward Elgar Publishing
Bhatti SS, Sambyal V, Singh J, Nagpal AK (2017) Analysis of soil characteristics of different land uses and metal bioaccumulation in wheat grown around rivers: possible human health risk assessment. Environ Dev Sustain 19(2):571–588
Bhatti SS, Kumar V, Kumar A, Gouzos J, Kirby J, Singh J, Sambyal V, Nagpal AK (2018) Potential ecological risks of metal(loid)s in riverine floodplain soils. Ecotoxicol Environ Saf 164:722–731
Bhatti SS, Kumar V, Kumar A, Kirby JK, Gouzos J, Correll R, Singh J, Sambyal V, Nagpal AK (2020) Potential carcinogenic and non-carcinogenic health hazards of metal(loid)s in food grains. Environ Sci Pollut Res 27:17032–17042
Bielecka K, Kurtek W, Banas D, Kubala-Kukus A, Braziewicza J, Majewska U, Pajek M, Wudarczyk-Mo¢ko J, Stabrawa I (2014) X-ray diffraction and elemental analysis of medical and environmental samples. Acta Phys Pol A 125:911–918
Bonnet M, Camares O, Veisseire P (2000) Effect of zinc and influence of Acremonium loliion growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J Exp Bot 51:945–953
C.So.Q.Gs. (2007) Canadian Soil quality guidelines for the protection of environmental and human health, summary tables, chap 7
Cabrera GL, Rodriguez DMG (1999) Genotoxicity of soil from farmland irrigated with wastewater using three plant bioassays. Mut Res 426:211–214
Campbell LM, Fisk AT, Wang X, Kock G, Muir DCG (2005) Evidence for biomagnification of rubidium in freshwater and marine food webs. Can J Fish Aquat Sci 62:1161–1167
Chandrasekaran A, Ravisankar R, Harikrishnan N, Satapathy KK, Prasad MVR, Kanagasabapathy KV (2015) Multivariate statistical analysis of heavy metal concentration in soils of Yelagiri Hills, Tamilnadu, India-Spectroscopical approach. Spectrochim Acta A Mol Biomol Spectrosc 137:589–600
China National Environmental Monitoring Center (CNEMC) (1990) The backgrounds of soil environment in China. China Environmental Science Press, Beijing (in Chinese)
Cobela-Garcia A, Prego R (2003) Heavy metal sedimentary record in a Glacian Ria (NW Spain): background values and recent contamination. Mar Pollut Bull 46:1253–1262
Denkhaus E, Salnikow K (2002) Nickel essentiality, toxicity, and carcinogenicity. Crit Rev Oncol/Hematol 42: 35–56
Faure G, Mensing TM (2005) Isotopes: principles and applications. Wiley, Hoboken
Funtua (1996) Application of the transmission emission method in EDXRF for the determination of trace element in geological and biological materials. J Trace Microprobe Tech 17:293–297
Gastaldo J, Viau M, Bencokova Z, Joubert A, Charvet A, Balosso J, Foray N (2007) Lead contamination results in late and slowly repairable DNA double-strand breaks and impacts upon the ATM-dependent signaling pathways. Toxicol Lett 173:201–214
Goldhaber MB, Lee RC, Hatch JR, Pashin JC, Treworgy J (2003) Role of large scale fluid-flow in subsurface arsenic enrichment. Arsenic in ground water. Springer, Bostn, MA, pp 127–164
Gowd SS, Govil PK (2008) Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu. India Environ Monit Assess 136:197–207
Gowd SS, Reddy MR, Govil PK (2010) Assessment of heavy metal contamination in soil at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh. India J Hazard Mater 174:113–121
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hammann A, Ybañez LM, Isla MI, Hilal M (2020) Potential agricultural use of a sub product (olive cake) from olive oil industries composting with soil. J Pharm Pharmacogn Res 8:43–52
Hammer Ø, Harper DA, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):9
Heggtveit HA, Grice HC, Wiberg GS (1970) Cobalt cardiomyopathy. Experimental basis for the human lesion. Path Microbiol 35:110–113
Hu J, Lin B, Yuan M, Lao Z, Wu K, Zeng Y, Liang Z, Li H, Li Y, Zhu D, Liu J (2019) Trace metal pollution and ecological risk assessment in agricultural soil in Dexing Pb/Zn mining area. China Environ Geochem Health 41(2):967–980
Ippolito J (2006) Biosolids affect soil barium in a dryland wheat agro-ecosystem. J Environ Qual 35:23–33
Jabeen S, Shah MT, Khan S, Hayat MQ (2010) Determination of major and trace elements in ten important folk therapeutic plants of Haripur basin. Pakistan J Med Plant Res 4:559–566
Juarez-Santacruz L, Garcia-Nieto E, Costilla-Salazar R, Garcia-Gallegos E, Coronel-Olivares C, Gomez-Camarillo M, Gaytan-Oyarzun J (2013) Assessment of the genotoxic potential of sediments contaminated with POPs and agricultural soils using Vicia faba micronucleus assay. Soil Sediment Contam 22:288–300
Kabata-Pendias A (2004) Soil-plant transfer of heavy metals-an environmental issue. Geoderma 122:143–149
Kabata-Pendias A, Pendias H (1999) Biogeochemistry 504 of trace elements, 2nd edn. PWN, Warsaw (In Polish)
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22(18):13772–13799
Kong MS, Ma TH (1999) Genotoxicity of contaminated soil and shallow well water detected by plant bioassays. Mutat Res 426(2):221–226
Kupper H, Kupper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth Res 58:125–133
Larocque ACL, Rasmuseen PE (1998) An overview of trace metals in the environment: mobilization to remediation. Environ Geol 33:85–91
Levan A (1938) The effect of colchicine on root mitosis in Allium. Hereditas 24:471–486
Loska K, Wiechuła D, Korus I (2004) Metal contamination of farming soils affected by industry. Environ Int 30(2):159–165
Lu X, Wanga L, Li LY, Lei K, Huang L, Kang D (2010) Multivariate statistical analysis of heavy metals in street dust of Baoji. NW China J Haz Mater 173:744–749
Ma Y, Hooda PS (2010) Chromium, nickel and cobalt. Trace Elem Soils 13:461–480
Ma L, Yang Z, Li L, Wang L (2016) Source identification and risk assessment of heavy metal contaminations in urban soils of Changsha, a mine-impacted city in Southern China. Environ Sci Pollut Res 23(17):17058–17066
Machender G, Dhakate R, Prasanna L, Govil PK (2011) Assessment of heavy metal contamination in soils around Balanagar industrial area, Hyderabaad. India Environ Earth Sci 63:945–953
Maksimovic I, Kastori R, Putnik-Delic M, Borišev M (2014) Effect of yttrium on photosynthesis and water relations in young maize plants. J Rare Earths 32(4):372–378
Marques CC, Nunes AC, Pinheiro T, Lopes PA, Santos MC, Viegas CAM, Ramalhinho MG, Mathias ML (2006) An assessment of time dependent effects of lead exposure in Algerian mice (Mus spretus) using different methodological approaches. Biol Trace Elem Res 109:75–89
Menzie C, Southworth B, Stephenson G, Feisthauer N (2008) The importance of understanding the chemical form of a metal in the environment: the case of barium sulfate (barite). Hum Ecol Risk Assess 14:974–991
Morinville A, Maysinger D, Shaver A (1998) From Vanadis to Atropos: vanadium compounds as pharmacological tools in cell death signaling. Trends Pharmacol Sci 19:452–460
Mudgal V, Madaan N, Mudgal A, Singh RB, Mishra S (2010) Effect of toxic metals on human health. Open Nutraceuticals J 3:94–99
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. GeoJournal 2:108–118
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216
Rigo AA, Cezaro AMD, Muenchen DK, Martinazzo J, Manzoli A, Steffens J, Steffens C (2020) Heavy metals detection in river water with cantilever nanobiosensor. J Environ Sci Health B 55(3):239–249
Ruggeberg A, Fietzke J, Liebetrau V, Eisenhauer A, Dullo W, Freiwald A (2008) Stable strontium isotopes (d88Sr/86Sr) in cold-water corals-a new proxyfor reconstruction of intermediate ocean water temperatures. Earth Planet Sci Lett 269:570–575
Sax K, Sax HJ (1968) Possible hazards of some food additives, beverages and insecticides. Idengakuzasshi
Shahid M, Ferrand E, Schreck E, Dumat C (2013) Behavior and impact of zirconium in the soil-plant system: plant uptake and phytotoxicity. In: Reviews of environmental contamination and toxicology, vol 221. Springer, New York, NY, pp 107–127
Shalan MG, Mostafa MS, Hassouna MM, Hassab El-Nabi SE, El-Rafaie A (2005) Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 206:1–15
Sharma S, Nagpal AK, Kaur I (2019) Appraisal of heavy metal contents in groundwater and associated health hazards posed to human population of Ropar wetland, Punjab, India and its environs. Chemosphere 227:179–190
Singh N, Kaur M, Katnoria JK (2017) Spatial and temporal heavy metal distribution and surface water characterization of Kanjli Wetland (a Ramsar site), India using different indices. Bull Environ Contam Toxicol 99(6):735–742
Soodan RK, Katnoria JK, Nagpal A (2014a) Allium cepa root chromosomal aberration assay: an efficient test system for evaluating genotoxicity of agricultural soil. Int J Sci Res 3:245–250
Soodan RK, Pakade YB, Nagpal A, Katnoria JK (2014b) Analytical techniques for estimation of heavy metals in soil ecosystem: a tabulated review. Talanta 125:405–410
Souza TS, Hencklein FA, Angelis DF, Fontanetti CS (2013) Clastogenicity of landfarming soil treated with sugar cane vinasse. Environ Monit Assess 185:1627–1636
Stern BR, Solioz M, Krewski D, Aggett P, Tar-Ching AW, Baker S, Crump K, Dourson M, Haber L, Hertzberg R, Keen C, Meek B, Rudenko L, Schoeny R, Slob W, Starr T (2007) Copper and human health: biochemistry, genetics, and strategies for modeling dose-response relationships. J Toxicol Environ Health 10:157–222
Stiborova M, Ditrichova M, Brezinova A (1988) Mechanism of action of Cu+2, Co+2 and Zn+2 on ribulose 1–5 biphosphate carboxylase from barley (Hordeum vulgare L.). Photosynthetica 22:161–167
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream, Oahu. Hawaii Environ Geol 39:611–627
Taylor SR, Mclennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Tsonko T, Fernando JCL (2012) Zinc in plants—an overview. Emir J Food Agric 24(4):322–333
Vachirapatama N, Dicinoski G, Townsend AT, Haddad PR (2002) Determination of vanadium as PAR-hydrogen peroxide complex in fertilisers by ion-interaction RP-HPLC. J Chromatogr A 956:221–227
Wang W, Zhou MF, Yan DP, Li L, Malpas J (2013) Detrital zircon record of Neoproterozoic active-margin sedimentation in the eastern Jiangnan Orogen. South China Precambrian Res 235:1–19
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
Welch AH, Westjohn DB, Helsel DR, Wanty RB (2000) Arsenic in ground water of the United States: Occurence and geochemistry. Groundwater 38(4):589
Yusuf AZ, Halima SA, Zakir A, Abdullahi M, Sani K (2014) X-ray fluorescence: an accessible and effective technique for evaluation of hazards and economic potential associated with important mineral deposits in Nigeria. J Geol Min Res 6:13–17
Zhang D, Wang XM, Li TF, Luo XQ, Wu W, Nori F, You JQ (2015) Cavity quantum electrodynamics with ferromagnetic magnons in a small yttrium-iron-garnet sphere. NPJ Quant Inform 1(1): 1–6
Zhang H, Mao Z, Huang K, Wang X, Cheng L, Zeng L, Zhou Y, Jing T (2019) Multiple exposure pathways and health risk assessment of heavy metal (loid) s for children living in fourth-tier cities in Hubei Province. Environ Int 129:517–524
Acknowledgements
Authors acknowledge University Grants Commission, New Delhi, for financial assistance under DRS and BSR programs; and Rajiv Gandhi National Fellowship; and Head, Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar for laboratory facilities.
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Vaneet Kumar and Sandip Singh Bhatti have equal first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, V., Bhatti, S.S. & Nagpal, A.K. Assessment of Metal(loid) Contamination and Genotoxic Potential of Agricultural Soils. Arch Environ Contam Toxicol 81, 272–284 (2021). https://doi.org/10.1007/s00244-021-00874-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00874-8