Abstract
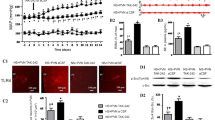
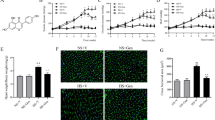
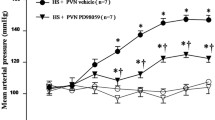
Long-term maternal salt intake induces the hypertension in offspring. Numerous studies have also indicated that high-salt diet causes the inflammation and an imbalance in neurotransmitters in the paraventricular nucleus (PVN) which increases the blood pressure and sympathetic activity. This study aimed to explore whether maternal salt intake induces hypertension in their male offspring by increasing the inflammation and changing the neurotransmitters balance in the paraventricular nucleus of offspring. This study includes two parts: Part I to explore the effect of high-salt diet on pregnant rats and the changes in inflammation and neurotransmitters in their male offspring PVN; Part II to reveal the influence on their offspring of bilateral PVN infusion of c-Src inhibitor dasatinib (DAS) in pregnant rats fed a high-salt diet. Maternal high-salt diet intake during copulation, pregnancy, and lactation impacted the offspring mean arterial pressure (MAP) and elevated the offspring PVN levels of p-Src, proinflammatory cytokines, and excitatory neurotransmitters. Bilateral PVN infusion of a c-Src inhibitor combined with maternal high-salt diets decreased MAP in the offspring. The infusion was also shown to suppress the Src-induced MAPK/NF-κB signaling pathway (p38 MAPK, JNK, Erk1/2), which attenuates inflammatory reactions. Finally, bilateral PVN infusion of the Src inhibitor in pregnant rat with high-salt diets improved the levels of inhibitory neurotransmitters in offspring PVN, which restored the excitatory-inhibitory neurotransmitter balance in male offspring. High-salt diets increase sympathetic activity and blood pressure in adult offspring, probably by activating the c-Src/MAPKs/NF-κB signaling pathway-induced inflammation. Moreover, NF-κB disrupts the downstream excitatory-inhibitory neurotransmitter balance in the PVN of male offspring.









Similar content being viewed by others
References
Wu, J., Li, N., Liu, Y., Li, W., He, A., Zhu, D., Feng, X., Liu, B., Shi, R., Zhang, Y., Lv, J., & Xu, Z. (2017). Maternal high salt diet altered Adenosine-mediated vasodilatation via PKA/BK channel pathway in offspring rats. Molecular Nutrition & Food Research, 61, 1600963.
Maruyama, K., Kagota, S., Van Vliet, B. N., Wakuda, H., & Shinozuka, K. (2015). A maternal high salt diet disturbs cardiac and vascular function of offspring. Life Sciences, 136, 42–51.
Boegehold, M. A. (2002). Microvascular structure and function in salt-sensitive hypertension. Microcirculation, 9, 225–241.
Blaustein, M. P., Leenen, F. H., Chen, L., Golovina, V. A., Hamlyn, J. M., Pallone, T. L., Van Huysse, J. W., Zhang, J., & Wier, W. G. (2012). How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. American Journal of Physiology-Heart and Circulatory Physiology, 302, H1031-1049.
Seravalli, P., de Oliveira, I. B., Zago, B. C., de Castro, I., Veras, M. M., Alves-Rodrigues, E. N., & Heimann, J. C. (2016). High and low salt intake during pregnancy: Impact on cardiac and renal structure in newborns. PloS One, 11, e0161598.
Liu, Y., Qi, L., Wu, J., Xu, T., Yang, C., Chen, X., Lv, J., & Xu, Z. (2018). Prenatal high-salt diet impaired vasodilatation with reprogrammed renin-angiotensin system in offspring rats. Journal of Hypertension, 36, 2369–2379.
Ding, Y., Lv, J., Mao, C., Zhang, H., Wang, A., Zhu, L., Zhu, H., & Xu, Z. (2010). High-salt diet during pregnancy and angiotensin-related cardiac changes. Journal of Hypertension, 28, 1290–1297.
Velten, M., Heyob, K. M., Wold, L. E., & Rogers, L. K. (2018). Perinatal inflammation induces sex-related differences in cardiovascular morbidities in mice. American Journal of Physiology-Heart and Circulatory Physiology, 314, H573–H579.
Pezeshki, Z., Eshraghi-Jazi, F., & Nematbakhsh, M. (2014). Vascular response to graded angiotensin II infusion in offspring subjected to high-salt drinking water during pregnancy: The effect of blood pressure, heart rate, urine output, endothelial permeability, and gender. International Journal of Vascular Medicine, 2014, 876527.
Sullivan, J. C. (2008). Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 294, R1220-1226.
Reynolds, C. M., Vickers, M. H., Harrison, C. J., Segovia, S. A., & Gray, C. (2014). High fat and/or high salt intake during pregnancy alters maternal meta-inflammation and offspring growth and metabolic profiles. Physiological Reports, 2, e12110.
Wang, M. L., Yu, X. J., Li, X. G., Pang, D. Z., Su, Q., Saahene, R. O., Li, H. B., Mao, X. Y., Liu, K. L., Fu, L. Y., Li, Y., Zhu, G. Q., & Kang, Y. M. (2018). Blockade of TLR4 within the paraventricular nucleus attenuates blood pressure by regulating ROS and inflammatory cytokines in prehypertensive rats. American Journal of Hypertension, 31, 1013–1023.
Wang, M. L., Kang, Y. M., Li, X. G., Su, Q., Li, H. B., Liu, K. L., Fu, L. Y., Saahene, R. O., Li, Y., Tan, H., & Yu, X. J. (2018). Central blockade of NLRP3 reduces blood pressure via regulating inflammation microenvironment and neurohormonal excitation in salt-induced prehypertensive rats. Journal of Neuroinflammation, 15, 95.
Zhang, D. D., Liang, Y. F., Qi, J., Kang, K. B., Yu, X. J., Gao, H. L., Liu, K. L., Chen, Y. M., Shi, X. L., Xin, G. R., Fu, L. Y., Kang, Y. M., & Cui, W. (2019). Carbon monoxide attenuates high salt-induced hypertension while reducing pro-inflammatory cytokines and oxidative stress in the paraventricular nucleus. Cardiovascular Toxicology, 19, 451–464.
Ngarashi, D., Fujikawa, K., Ferdaus, M. Z., Zahid, H. M., Ohara, H., & Nabika, T. (2019). Dual inhibition of NADPH oxidases and xanthine oxidase potently prevents salt-induced stroke in stroke-prone spontaneously hypertensive rats. Hypertension Research: Official Journal of the Japanese Society of Hypertension, 42, 981–989.
Zheng, X., Li, X., Chen, M., Yang, P., Zhao, X., Zeng, L., OuYang, Y., Yang, Z., & Tian, Z. (2019). The protective role of hawthorn fruit extract against high salt-induced hypertension in Dahl salt-sensitive rats: Impact on oxidative stress and metabolic patterns. Food & Function, 10, 849–858.
Yu, X. J., Miao, Y. W., Li, H. B., Su, Q., Liu, K. L., Fu, L. Y., Hou, Y. K., Shi, X. L., Li, Y., Mu, J. J., Chen, W. S., Cui, W., Zhu, G. Q., Ebenezer, P. J., Francis, J., & Kang, Y. M. (2019). Blockade of endogenous angiotensin-(1–7) in hypothalamic paraventricular nucleus attenuates high salt-induced sympathoexcitation and hypertension. Neuroscience Bulletin, 35, 47–56.
Banek, C. T., Gauthier, M. M., Van Helden, D. A., Fink, G. D., & Osborn, J. W. (2019). Renal inflammation in DOCA-salt hypertension. Hypertension, 73, 1079–1086.
Brugge, J. S., Cotton, P. C., Queral, A. E., Barrett, J. N., Nonner, D., & Keane, R. W. (1985). Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature, 316, 554–557.
Barnekow, A., & Gessler, M. (1986). Activation of the pp60c-src kinase during differentiation of monomyelocytic cells in vitro. The EMBO Journal, 5, 701–705.
Callera, G. E., Touyz, R. M., Tostes, R. C., Yogi, A., He, Y., Malkinson, S., & Schiffrin, E. L. (2005). Aldosterone activates vascular p38MAP kinase and NADPH oxidase via c-Src. Hypertension, 45, 773–779.
Touyz, R. M., Yao, G., & Schiffrin, E. L. (2003). c-Src induces phosphorylation and translocation of p47phox: Role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology, 23, 981–987.
Touyz, R. M., He, G., Wu, X. H., Park, J. B., Mabrouk, M. E., & Schiffrin, E. L. (2001). Src is an important mediator of extracellular signal-regulated kinase 1/2-dependent growth signaling by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients. Hypertension, 38, 56–64.
Massip Copiz, M. M., & Santa Coloma, T. A. (2016). c-Src and its role in cystic fibrosis. European Journal of Cell Biology, 95, 401–413.
Song, C., Hong, Y. H., Park, J. G., Kim, H. G., Jeong, D., Oh, J., Sung, G. H., Hossain, M. A., Taamalli, A., Kim, J. H., & Cho, J. Y. (2019). Suppression of Src and Syk in the NF-kappaB signaling pathway by Olea europaea methanol extract is leading to its anti-inflammatory effects. Journal of Ethnopharmacology, 235, 38–46.
Xu, B., & Li, H. (2015). Brain mechanisms of sympathetic activation in heart failure: Roles of the reninangiotensin system, nitric oxide and proinflammatory cytokines (Review). Molecular Medicine Reports, 12, 7823–7829.
Leenen, F. H. (2014). Actions of circulating angiotensin II and aldosterone in the brain contributing to hypertension. American Journal of Hypertension, 27, 1024–1032.
Arenas, Y. M., Cabrera-Pastor, A., Juciute, N., Mora-Navarro, E., & Felipo, V. (2020). Blocking glycine receptors reduces neuroinflammation and restores neurotransmission in cerebellum through ADAM17-TNFR1-NF-kappabeta pathway. Journal of Neuroinflammation, 17, 269.
Karki, P., Hong, P., Johnson, J., Jr., Pajarillo, E., Son, D. S., Aschner, M., & Lee, E. Y. (2018). Arundic acid increases expression and function of astrocytic glutamate transporter EAAT1 via the ERK, Akt, and NF-kappaB pathways. Molecular Neurobiology, 55, 5031–5046.
Lozic, M., Sarenac, O., Murphy, D., & Japundzic-Zigon, N. (2018). Vasopressin, central autonomic control and blood pressure regulation. Current Hypertension Reports, 20, 11.
Kang, Y. M., Yang, Q., Yu, X. J., Qi, J., Zhang, Y., Li, H. B., Su, Q., & Zhu, G. Q. (2014). Hypothalamic paraventricular nucleus activation contributes to neurohumoral excitation in rats with heart failure. Regenerative Medicine Research, 2, 2.
Sun, Y., Sun, B., & He, R. (2017). Effect of the changes of NMDA receptor in hypothalamic paraventricular nucleus on cardiac function and sympathetic nervous activity in rats with heart failure. Biochemical and Biophysical Research communications, 493, 1336–1341.
Kang, Y. M., Zhang, A. Q., Zhao, X. F., Cardinale, J. P., Elks, C., Cao, X. M., Zhang, Z. W., & Francis, J. (2011). Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Research in Cardiology, 106, 473–483.
Kang, Y. M., He, R. L., Yang, L. M., Qin, D. N., Guggilam, A., Elks, C., Yan, N., Guo, Z., & Francis, J. (2009). Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovascular Research, 83, 737–746.
Li, H. B., Qin, D. N., Suo, Y. P., Guo, J., Su, Q., Miao, Y. W., Sun, W. Y., Yi, Q. Y., Cui, W., Cheng, K., Zhu, G. Q., & Kang, Y. M. (2015). Blockade of salusin-beta in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy in salt-induced hypertensive rats. Journal of Cardiovascular Pharmacology, 66, 323–331.
Su, Q., Qin, D. N., Wang, F. X., Ren, J., Li, H. B., Zhang, M., Yang, Q., Miao, Y. W., Yu, X. J., Qi, J., Zhu, Z., Zhu, G. Q., & Kang, Y. M. (2014). Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicology and Applied Pharmacology, 276, 115–120.
Li, H. B., Qin, D. N., Ma, L., Miao, Y. W., Zhang, D. M., Lu, Y., Song, X. A., Zhu, G. Q., & Kang, Y. M. (2014). Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicology and Applied Pharmacology, 279, 141–149.
Elks, C. M., Reed, S. D., Mariappan, N., Shukitt-Hale, B., Joseph, J. A., Ingram, D. K., & Francis, J. (2011). A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress. PloS ONE, 6, e24028.
Xia, W. J., Xu, M. L., Yu, X. J., Du, M. M., Li, X. H., Yang, T., Li, L., Li, Y., Kang, K. B., Su, Q., Xu, J. X., Shi, X. L., Wang, X. M., Li, H. B., & Kang, Y. M. (2021). Antihypertensive effects of exercise involve reshaping of gut microbiota and improvement of gut-brain axis in spontaneously hypertensive rat. Gut Microbes, 13, 1–24.
Krum, H., Sobotka, P., Mahfoud, F., Bohm, M., Esler, M., & Schlaich, M. (2011). Device-based antihypertensive therapy: Therapeutic modulation of the autonomic nervous system. Circulation, 123, 209–215.
Xie, L., Mao, X., Jin, K., & Greenberg, D. A. (2013). Vascular endothelial growth factor-B expression in postischemic rat brain. Vascular Cell, 5, 8.
Li, H. B., Qin, D. N., Cheng, K., Su, Q., Miao, Y. W., Guo, J., Zhang, M., Zhu, G. Q., & Kang, Y. M. (2015). Central blockade of salusin beta attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Scientific Reports, 5, 11162.
Cui, L., Meng, Y., Xu, D., Feng, Y., Chen, G., Hu, B., Feng, G., & Yin, L. (2013). Analysis of the metabolic properties of maintenance hemodialysis patients with glucose-added dialysis based on high performance liquid chromatography quadrupole time-of-flight mass spectrometry. Therapeutics and Clinical Risk Management, 9, 417–425.
Barber, M., Kasturi, B. S., Austin, M. E., Patel, K. P., MohanKumar, S. M., & MohanKumar, P. S. (2003). Diabetes-induced neuroendocrine changes in rats: Role of brain monoamines, insulin and leptin. Brain Research, 964, 128–135.
Contreras, R. J. (1993). High NaCl intake of rat dams alters maternal behavior and elevates blood pressure of adult offspring. The American Journal of Physiology, 264, R296-304.
Mao, C., Liu, R., Bo, L., Chen, N., Li, S., Xia, S., Chen, J., Li, D., Zhang, L., & Xu, Z. (2013). High-salt diets during pregnancy affected fetal and offspring renal renin-angiotensin system. The Journal of Endocrinology, 218, 61–73.
Schmidt-Pogoda, A., Strecker, J. K., Liebmann, M., Massoth, C., Beuker, C., Hansen, U., Konig, S., Albrecht, S., Bock, S., Breuer, J., Sommer, C., Schwab, N., Wiendl, H., Klotz, L., & Minnerup, J. (2018). Dietary salt promotes ischemic brain injury and is associated with parenchymal migrasome formation. PloS ONE, 13, e0209871.
Rust, P., & Ekmekcioglu, C. (2017). Impact of salt intake on the pathogenesis and treatment of hypertension. Advances in Experimental Medicine and Biology, 956, 61–84.
Leandro, S. M., Furukawa, L. N., Shimizu, M. H., Casarini, D. E., Seguro, A. C., Patriarca, G., Coelho, M. S., Dolnikoff, M. S., & Heimann, J. C. (2008). Low birth weight in response to salt restriction during pregnancy is not due to alterations in uterine-placental blood flow or the placental and peripheral renin-angiotensin system. Physiology & Behavior, 95, 145–151.
Yang, Q., Yu, X.-J., Su, Q., Yi, Q.-Y., Song, X.-A., Shi, X.-L., Li, H.-B., Qi, J., Zhu, G.-Q., & Kang, Y.-M. (2019). Blockade of c-Src within the paraventricular nucleus attenuates inflammatory cytokines and oxidative stress in the mechanism of the TLR4 signal pathway in salt-induced hypertension. Neuroscience Bulletin, 36, 385.
Moura, E., Afonso, J., Serrao, M. P., & Vieira-Coelho, M. A. (2009). Effect of clonidine on tyrosine hydroxylase activity in the adrenal medulla and brain of spontaneously hypertensive rats. Basic & Clinical Pharmacology & Toxicology, 104, 113–121.
Basting, T., Xu, J., Mukerjee, S., Epling, J., Fuchs, R., Sriramula, S., & Lazartigues, E. (2018). Glutamatergic neurons of the paraventricular nucleus are critical contributors to the development of neurogenic hypertension. The Journal of Physiology, 596, 6235–6248.
Tsuda, K., & Masuyama, Y. (1991). Presynaptic regulation of neurotransmitter release in hypertension. Clinical and Experimental Pharmacology & Physiology, 18, 455–467.
Maes, M. (1995). Evidence for an immune response in major depression: A review and hypothesis. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 19, 11–38.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 81800373, 82070439, 81770426, 81700373, 82070440), Shaanxi Basic Research on Natural Science (Nos. 2019JQ-263, 2020JM-079), the China Postdoctoral Science Foundation (No. 2019M660259).
Author information
Authors and Affiliations
Contributions
QS, XJY, and YMK designed the study. QS, QY, HBL, and WJX performed all experiments. QY, HBL, XMW, XJY, and QS also performed, checked the data analysis, and drafted the manuscript. QY, XMW, and KLL participated in data analysis. XMW helped to check the responses and revised manuscript. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None of the listed authors has any financial or other interest that could be of conflict.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Su, Q., Yu, XJ., Yang, Q. et al. Inhibition of Maternal c-Src Ameliorates the Male Offspring Hypertension by Suppressing Inflammation and Neurotransmitters in the Paraventricular Nucleus. Cardiovasc Toxicol 21, 820–834 (2021). https://doi.org/10.1007/s12012-021-09672-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09672-z