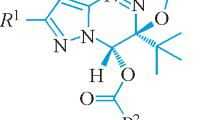
The structures of 7-amino-3-tert-butyl-4-oxo-1,4-dihydropyrazolo[5,1-c][1,2,4]triazine-8-carbonitrile and the corresponding 8-ethyl carboxylate were studied by xray crystal diffraction analysis. Both molecules have a planar conjugated heteronucleus with a marked tendency to form intra- and intermolecular hydrogen bonds. The molecular bond lengths and angles in the single crystals were determined.
Similar content being viewed by others
References
E. K. Voinkov, R. A. Drokin, E. N. Ulomsky, O. N. Chupakhin, V. N. Charushin, and V. L. Rusinov, Chem. Heterocycl. Comp., 56, No. 10, 1254–1273 (2020); https://doi.org/10.1007s1059302808z.
S. M. Ivanov, J. Heterocycl. Chem., 57, 3510–3530 (2020), https://doi.org/https://doi.org/10.1002/jht.4071.
K. V. Savateev, E. N. Ulomsky, I. I. Butorin, V. N. Charushin, V. L. Rusinov, and O. N. Chupakhin, Russ. Chem. Rev., 87, 636–669 (2018); https://doi.org/https://doi.org/10.1070/RCR4792.
S. M. Ivanov, V. F. Traven, and M. E. Minyaev, Struct. Chem., 31, No. 4, 1457–1470 (2020); https://doi.org/https://doi.org/10.1007/s11224020015339.
S. M. Ivanov, L. M. Mironovich, and M. E. Minyaev, Phosphor. Sulfur Sil. Rel. Elem., 195, No. 8, 666–676 (2020), https://doi.org/https://doi.org/10.1080/10426507.2020.1712395.
S. M. Ivanov, K. A. Lyssenko, and V. F. Traven, Russ. Chem. Bull., 69, No. 4, 731–738 (2020); https://doi.org/https://doi.org/10.1007/s1117202028254.
A. N. Izmest'ev, D. A. Vasileva, E. K. Melnikova, N. G. Kolotyrkina, I. A. Borisova, A. N. Kravchenko, and G. A. Gazieva, New J. Chem., 43, 1038–1052 (2019); https://doi.org/https://doi.org/10.1039/C8NJ05058A.
M. C. Schulze, B. L. Scott, and D. E. Chavez, J. Mat. Chem. A, 3, 17963–17965 (2015); https://doi.org/https://doi.org/10.2039/C5TA05291B.
S. M. Ivanov, L. A. Rodinovskaya, A. A. Shestopalov, and L. M. Mironovich, Russ. Chem. Bull., 66, No. 6, 1126–1130 (2017); https://doi.org/https://doi.org/10.1007/s111720171865x.
L. M. Mironovich, S. M. Ivanov, and E. D. Daeva, Russ. J. Org. Chem., 55, No. 7, 958–963 (2019); https://doi.org/https://doi.org/10.1134/S1070428019070066.
L. M. Mironovich, S. M. Ivanov, and E. D. Daeva, Russ. J. Org. Chem., 54, No. 12, 1825–1830 (2018); https://doi.org/https://doi.org/10.1134/S10704280181120151.
S. M. Ivanov, L. M. Mironovich, P. N. Solyev, L. A. Rodinovskaya, and A. M. Shestopalov, J. Heterocycl. Chem., 55, No. 2, 545–550 (2018); https://doi.org/https://doi.org/10.1002/jhet.3064.
Bruker. APEXIII, Bruker AXS Inc., Madison, Wisconsin, USA (2018).
L. Krause, R. HerbstIrmer, G. M. Sheldrick, and D. Stalke, J. Appl. Cryst., 48, 3–10 (2015); https://doi.org/https://doi.org/10.1107/S1600576714022985.
G. M. Sheldrick, Acta Cryst., A71, 3–8 (2015); https://doi.org/https://doi.org/10.1107/S2053273314026370.
G. M. Sheldrick, Acta Cryst., C71, 3–8 (2015); https://doi.org/https://doi.org/10.1107/S2053229614024218.
L. M. Mironovich and M. V. Kostina, Chem. Heterocycl. Comp., 47, No. 10, 1286–1289 (2011); https://doi.org/https://doi.org/10.1007/s1059301209047.
L. M. Mironovich and M. V. Kostina, Russ. J. Gen. Chem., 83, No. 1, 152–153 (2013); https://doi.org/https://doi.org/10.1134/S1070363213010337.
L. M. Mironovich and D. V. Shcherbinin, Russ. J. Org. Chem., 50, No. 12, 1860–1862 (2014); https://doi.org/https://doi.org/10.1134/S1070428014120306.
S. M. Ivanov, L. M. Mironovich, L. A. Rodinovskaya, and A. M. Shestopalov, Russ. Chem. Bull., 67, No. 8, 1487–1491 (2018); https://doi.org//10.1007/s111720182243z.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 88, No. 3, pp. 408–414, May–June, 2021.
Rights and permissions
About this article
Cite this article
Mironovich, L.M., Ivanov, S.M. Crystal Structure of 7-Amino-8-R-3-tert-Butyl-4-Oxo-1,4-Dihydropyrazolo[5,1-c][1,2,4]Triazines. J Appl Spectrosc 88, 528–534 (2021). https://doi.org/10.1007/s10812-021-01204-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-021-01204-5