Abstract
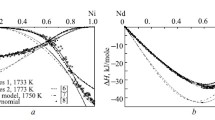
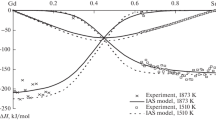
The enthalpies of mixing of Ni–Ln NiPr melts (0 < xNi < 0.6) are studied via isoperibol calorimetry at 1600 ± 1 K. The enthalpies of mixing for Ni–Pr melts are characterized by high exothermic values. The minimum enthalpy of mixing for the melts of the considered system is −35.3 ± 0.9 kJ/mol at xNi = 0.6. The activities of the components and the molar fractions of the most stable associates in melts of the Ni–Pr system are estimated using the model of ideal associated solutions (IAS). Literature data (enthalpies of formation of PrmNin compounds, phase diagram of the Ni–Pr system) and the obtained thermochemical data are both used. Five associates are selected for calculations. It is shown that the activities of the components in the melts of this system display large negative deviations from Raoult’s law, and the PrNi2 associate predominates in them.






Similar content being viewed by others
REFERENCES
P. Rogl, in Handbook on the Physics and Chemistry of Rare Earths, Ed. by K. A. Gschneidner, Jr. and L. R. Eyring (North-Holland Physics, Amsterdam, 1987), Vol. 7, p. 1.
M. Huang and T. A. Lograsso, J. Alloys Compd. 395, 75 (2005).
M. Huang, D. Wu, K. W. Dennis, J. W. Anderegg, et al., Phase Equilib. 26, 209 (2005).
R. Vogel and W. Fülling, Metallforschung, No. 2, 97 (1947).
Y. Y. Pan and C. S. Cheng, in Proceedings of the Chinese National Symposium on Phase Diagrams, Kunming, September 1984.
G. Qi, Z. Li, K. Itagaki, and A. Yazawa, Mater. Trans. JIM 30, 583 (1989).
K. A. Gschneidner, Rare Earth Alloys, A Critical Review of the Alloy Systems of the Rare Earth, Scandium, and Yttrium Metals (Van Nostrand, Princeton, NJ, 1961).
K. H. J. Buschow and A. S. van der Goot, Less-Common. J. Met. 22, 419 (1970).
J. H. Wernick and S. Geller, Acta Crystallogr. 12, 662 (1959).
A. E. Dwigh, Trans. Am. Soc. Metals 53, 479 (1961).
D. Paccard and R. Pauthenet, C.R. Acad. Sci., Ser. A B 264, 1056 (1967).
J. H. Wernick and S. Geller, Trans. Metall. Soc. AIME 218, 866 (1960).
A. E. Dwight, R. A. Conner, Jr., and J. W. Downey, AD Report (U. S. Clearinghouse for Fed. Sci. Tech. Inform., 1965), p. 35.
S. C. Abrahams, J. L. Bernstein, R. C. Sherwood, et al., Phys. Chem. Solids 25, 1069 (1964).
R. E. Walline and W. E. Wallace, J. Chem. Phys. 41, 1587 (1964).
R. Lemaire and D. Paccard, Bull. Soc. Fr. Miner. Cristallogr. 90, 311 (1967).
F. Kissell, T. Tsuchida, and W. E. Wallace, J. Chem. Phys. 44, 4651 (1966).
G. L. Olcese, J. Less-Common. Met. 33, 71 (1973).
Y. Y. Pan and P. Nash, Bull. Alloy Phase Diagr. 10, 253 (1989).
Z. Du, D. Wang, and W. J. Zhang, J. Alloys Compd. 284, 206 (1999).
H. Okamoto, J. Phase Equilib. 26, 650 (2005).
Z. Rahou, K. Mahdouk, D. Moustain, et al., J. Alloys Compd. 620, 204 (2015).
S. S. Deodhar and P. J. Ficalora, Metall. Trans. A: Phys. Metall. Mater. Sci. 6, 1909 (1975).
A. Pasturel, C. Colinet, C. Allibert, et al., Phys. Status Solidi B 125, 101 (1984).
C. Colinet, A. Pasturel, and K. H. I. Buschow, Met. Trans. A 17, 777 (1986).
Qi Guo and O. L. Kleppa, Met. Mater. Trans. B 25, 73 (1994).
Qi Guo and O. L. Kleppa, J. Alloys Compd. 270, 212 (1998).
S. Delsante, R. Stifanese, and G. Borzone, J. Chem. Thermodyn. 65, 73 (2013).
I. V. Nikolaenko, J. Alloys Compd. 225, 474 (1995).
Y. Khan and D. Feldmann, J. Less-Common. Met. 33, 305 (1973).
T. Yamamoto, H. Inui, M. Yamaguchi, et al., Acta Mater. 45, 5213 (1997).
L. Lemort et al., J Alloys Compd. 509, S823 (2011).
K. Iwase, K. Sakaki, J. Matsuda, et al., Inorg. Chem. 50, 4548 (2011). dx.doi.org.https://doi.org/10.1021/ic200253w
M. Mardani, I. Fartushna, A. Khvan, et al., J. Alloys Compd. 781, 524 (2019).
I. Fartushna, M. Mardani, and I. Bajenova, et al., J. Alloys Compd. 845, 15635 (2020).
V. V. Berezutskii, M. A. Shevchenko, M. I. Ivanov, and V. S. Sudavtsova, Russ. J. Phys. Chem. A 88, 1463 (2014). https://doi.org/10.1134/S0036024414090064
A. T. Dinsdale, CALPHAD 15, 319 (1991).
M. Ivanov, V. Berezutski, N. Usenko, and N. Kotova, Int. J. Mater. Res. 104, 849 (2013).
V. S. Sudavtsova, M. A. Shevchenko, V. G. Kudin, et al., Poroshk. Metall., Nos. 1–2, 110 (2017).
I. V. Nikolaenko and M. A. Turchanin, Rasplavy 2 (6), 75 (1988).
J. Schott and F. Sommer, J. Less-Common. Met. 119, 307 (1986).
V. S. Sudavtsova, M. O. Shevchenko, M. Yu. Ivanov, et al., Poroshk. Metall., No. 9/10, 107 (2019).
V. V. Berezutskii and M. I. Ivanov, Poroshk. Metall., No. 7/8, 111 (2009).
M. Ivanov, V. Berezutski, N. Usenko, and N. Kotova, Int. J. Mater. Res. 108, 29 (2017). https://doi.org/10.3139/146.111445
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Bannov
Rights and permissions
About this article
Cite this article
Kudin, V.G., Romanova, L.A., Shevchenko, M.A. et al. Phase Equilibria and Thermodynamics of Ni–Pr Alloys. Russ. J. Phys. Chem. 95, 1295–1301 (2021). https://doi.org/10.1134/S0036024421070153
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421070153