Abstract
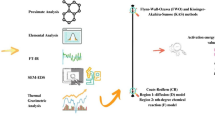
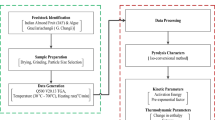
The present research aims towards the study of thermal degradation kinetics and thermodynamics of Arachis hypogaea shells (AHS) to evaluate its potential for bioenergy production. Physicochemical characterization, i.e., proximate, ultimate, compositional analysis, and higher heating value (HHV) were carried out in addition to thermogravimetric (TG) analysis. Physicochemical characterization revealed high volatile matter (75.2 wt.%) with considerably lower moisture, ash contents, and significantly higher HHV (17 MJ/kg). TG analysis of AHS was conducted from ambient to 800 °C at multiple heating rates (10, 15, and 25 °C/min) using nitrogen as carrier gas. TG and derivative thermogravimetric (DTG) analysis disclosed that the maximum degradation occurs in the temperature ranging from 150 to 450 °C (~64%). The iso-conversional methods that were employed to determine kinetic and thermodynamic parameters are Flynn-Wall-Ozawa (FWO), Kissinger-Akahira-Sunose (KAS), Starink, and Friedman. Average values of activation energies as calculated by these models were 175.05, 173.65, 171.83, 175.95 kJ/mol respectively. The values of pre-exponential factor (A0) lie in the magnitude of 109–1020 s−1. The calculated average values of Gibbs free energy (ΔG) by FWO, KAS, Starink, and Friedman were 154.61, 154.66, 154.70, 154.63 kJ/mol, respectively. The average change in enthalpy (ΔH) and change in entropy (ΔS) for the degradation process were in between 166.54–170.65 kJ/mol and 19.99–27.05 J/mol.K, respectively. Reaction mechanism estimation was done using the Z-plot method associated with the Criado method which confirmed that thermal degradation of AHS follows multiple reaction mechanisms. The results suggest that AHS has the potential to be effectively used for the generation of bioenergy.







Similar content being viewed by others
References
Rapier R (2020) Fossil fuels still supply 84 percent of the world’s energy-and other eye opener from BP’s annual review. Forbes https://www.forbes.com/sites/rrapier/2020/06/20/bp-review-new-highs-in-global-energy-consumption-and-carbon-emissions-in-2019/?sh=2d26a63766a1. Accessed 10 Apr 2021
USAID (2019) Greenhouse gas emissions factsheet: India. Climatelinks https://www.climatelinks.org/resources/greenhouse-gas-emissions-factsheet-india. Accessed 10 Apr 2021
Subramanian K (2019) Is India on track to meet its Paris commitments. Down to Earth https://www.downtoearth.org.in/blog/climate-change/is-india-on-track-to-meet-its-paris-commitments-67345. Accessed 10 Apr 2021
Jaiswal A (2019) The road from Paris: progress on India’s climate pledge. NRDC https://www.nrdc.org/experts/anjali-jaiswal/road-paris-indias-advancement-its-climate-pled. Accessed 10 Apr 2021
Burhenne L, Messmer J, Aicher T, Laborie MP (2013) The effects of biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J Anal Appl Pyrolysis 101:177–184
Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Lappas AA (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150
Bhoi PR, Ouedraogo AS, Soloiu V, Quirino R (2020) Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew Sust Energ Rev 121:109676
Gupta S, Gupta GK, Mondal MK (2019) Slow pyrolysis of chemically treated walnut shell for valuable products: effect of process parameters and in-depth product analysis. Energy 181:665–676
Chen X, Che Q, Li S, Liu Z, Yang H, Chen Y, Wang X, Shao J, Chen H (2019) Recent developments in lignocellulosic biomass catalytic fast pyrolysis: strategies for the optimization of bio-oil quality and yield. Fuel Process Technol 196:106180
Gupta GK, Gupta PK, Mondal MK (2019) Experimental process parameters optimization and in-depth product characterization for teak sawdust pyrolysis. Waste Manag 87:499–511
Abdelouahed L, Leveneur S, Vernieres-Hassimi L, Balland L, Taouk B (2017) Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J Therm Anal Calorim 129:1201–1213
Luo L, Guo X, Zhang Z, Chai M, Rahman MM, Zhang X, Cai J (2020) Insight into pyrolysis kinetics of lignocellulosic biomass: isoconversional kinetic analysis by the modified Friedman method. Energy Fuel 34(4):4874–4881
Statista Research Department (2021) Peanut consumption in India (2012–2018). https://www.statista.com/statistics/872360/india-peanut-consumption. Accessed 10 Apr 2021
Perea-Moreno MA, Manzano-Agugliaro F, Hernandez-Escobedo Q, Perea-Moreno AJ (2018) Peanut shell for energy: properties and its potential to respect the environment. Sustainability 10(9):3254
Simon P (2004) Isoconversional methods: fundamentals, meaning and application. J Therm Anal Calorim 76:123–132
Rueda-Ordóñez YJ, Tannous K (2015) Isoconversional kinetic study of the thermal decomposition of sugarcane straw for thermal conversion processes. Bioresour Technol 196:136–144
Mumbach GD, Alves JLF, Gomes da Silva JC, Domenico MD, Sena RFD, Marangoni C, Machado RAF, Bolzan A (2020) Pyrolysis of cocoa shell for its bioenergy potential: evaluating the kinetic triplet, thermodynamic parameters, and evolved gas analysis using TGA-FTIR. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01058-5
Alves JLF, Gomes da Silva JC, Domenico MD, Galdino WVDA, Andersen SLF, Alves RF, Sena RFD (2021) Exploring açaí seed (Euterpe oleracea) pyrolysis using multi-component kinetics and thermodynamics assessment towards its bioenergy potential. Bioenergy Res 14:209–225
Arenas CN, Navarro MV, Martinez JD (2019) Pyrolysis kinetics of biomass waste using isoconversional methods and the distributed activation energy model. Bioresour Technol 288:121485
Gupta S, Gupta GK, Mondal MK (2020) Thermal degradation characteristics, kinetics, thermodynamics and reaction mechanism analysis of pistachio shell pyrolysis for its bioenergy potential. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01104-2
Gupta GK, Mondal MK (2019) Kinetic and thermodynamic analysis of maize cob pyrolysis for its bioenergy potential using thermogravimetric analyzer. J Therm Anal Calorim 137:1431–1441
Mallick D, Poddar MK, Mahanta P, Moholkar VS (2018) Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour Technol 261:294–305
Sarkar JK, Wang Q (2020) Characterization of pyrolysis products and kinetic analysis of waste jute stick biomass. Processes 8(7):837
Yuan X, He T, Cao H, Yuan Q (2017) Cattle manure pyrolysis process: kinetic and thermodynamic analysis with isoconversional methods. Renew Energy 107:489–496
Gupta GK, Mondal MK (2018) Iso-conversional kinetic and thermodynamic studies of Indian sagwan sawdust pyrolysis for its bioenergy potential. Environ Prog Sustain Energy 38(4):13131
Singh S, Chakraborty JP, Mondal MK (2020) Intrinsic kinetics, thermodynamic parameters and reaction mechanism of non-isothermal degradation of torrefied Acacia nilotica using isoconversional methods. Fuel 259:116263
Kaur R, Gera P, Jha MK, Bhaskar T (2018) Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour Technol 250:422–428
Lanjewar R, Thakur LS, Parmar H, Varma AK, Hinge VK (2019) A review on physicochemical characterization and pyrolysis kinetics if biomass. International Journal of Management, Technology and Engineering 9(3):4595–4617
Huang YF, Lo SL (2020) Predicting heating value of lignocellulosic biomass based on elemental analysis. Energy 191:116501
Acikalin K (2021) Determination of kinetic triplet, thermal degradation behavior and thermodynamic properties for pyrolysis of a lignocellulosic biomass. Bioresour Technol 337:125438
Singh RK, Patil T, Sawarkar AN (2020) Pyrolysis of garlic husk biomass: physico-chemical characterization, thermodynamic and kinetic analyses. Bioresource Technology Reports 12(10055):8
Ceylan S, Topçu Y (2014) Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour Technol 156:182–188
Rathore NS, Pawar A, Pawar NL (2021) Kinetic analysis and thermal degradation study on wheat straw and its biochar from vacuum pyrolysis under non-isothermal conditions. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01360-w
Cai J, Xua D, Dong Z, Yu X, Yang Y, Banks SW, Bridgwater AV (2018) Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk. Renew Sust Energ Rev 82(3):2705–2715
Yang H, Yan R, Chen H, Zheng C, Lee DH, Liang DT (2005) In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuel 20:388–393
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74
Rasool T, Kumar S (2020) Kinetic and thermodynamic evaluation of pyrolysis of plant biomass using TGA. Material Today: Proceedings 21(4):2087–2095
Santos VO, Queiroz LS, Araujo RO, Ribeiro FCP, Guimarães MN, Costa CEF, Chaar JS, Souza LKC (2020) Pyrolysis of acai seed biomass: kinetics and thermodynamic parameters using thermogravimetric analysis. Bioresour Technol Reports 12:100553
Gouda N, Panda AK (2019) Determination of kinetic and thermodynamic parameters of thermal degradation of different biomasses for pyrolysis. Biocatal Agri Biotechnol 21:101315
Kumar M, Mishra PK, Upadhyay SN (2020) Thermal degradation of rice husk: effect of pre-treatment on kinetic and thermodynamic parameters. Fuel 268:117164
Kumar M, Shukla SK, Upadhyay SN, Mishra PK (2020) Analysis of thermal degradation of banana (Musa balbisiana) trunk biomass waste using iso-conversional models. Bioresour Technol 310:123393
Seo DK, Park SS, Hwang J, Yu TU (2010) Study of the pyrolysis of biomass using thermo-gravimetric analysis (TGA) and concentration measurements of the evolved species. J Anal Appl Pyrolysis 89(1):66–73
Maiti S, Purakayastha S, Ghosh B (2007) Thermal characterization of mustard straw and stalk in nitrogen at different heating rates. Fuel 86(10–11):1513–1518
Xiao R, Yang W, Cong X, Dong K, Xu J, Wang D, Yang X (2020) Thermogravimetric analysis and reaction kinetics of lignocellulosic biomass pyrolysis. Energy 201:117537
Dash M, Dasu VV, Mohanty K (2013) Non-isothermal kinetic study of three lignocellulosic biomass using model-free methods. J Renew Sustain Energy 5(6):063101
Dhyani V, Kumar J, Bhaskar T (2017) Thermal decomposition kinetics of sorghum straw via thermogravimetric analysis. Bioresour Technol 245:1122–1129
Mishra G, Kumar J, Bhaskar T (2015) Kinetic studies on the pyrolysis of pinewood. Bioresour Technol 182:282–288
Gajera ZR, Verma K, Tekade SP, Sawarkar AN (2020) Kinetics of co-gasification of rice husk biomass and high Sulphur petroleum coke with oxygen as gasifying medium via TGA. Bioresour Technol Reports 11:100479
Mehmood MA, Ye G, Luo H, Liu C, Malik S, Afzal I, Xu J, Ahmad MS (2017) Pyrolysis and kinetic analyses of camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour Technol 228:18–24
Ahmad MS, Mehmood MA, Al Ayed OS, Ye G, Luo H, Ibrahim M, Rashid U, Nehdi AI, Qadir G (2017) Kinetic analyses and pyrolytic behavior of para grass (Urochloa mutica) for its bioenergy potential. Bioresour Technol 224:708–713
Laougé ZB, Merdun H (2020) Pyrolysis and combustion kinetics of Sida cordifolia L. using thermogravimetric analysis. Bioresour Technol 299:122602
Varma AK, Mondal P (2016) Physicochemical characterization and kinetic study of pine needle for pyrolysis process. J Therm Anal Calorim 124:487–497
Cai JM, Bi LS (2009) Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J Therm Anal Calorim 98:325–330
Nam HV, Tam TT, Tho VDS (2019) Kinetic modelling of thermal decomposition of sugarcane bagasse in the inert gas environment. Vietnam J Chem 57(5):574–580
Doddapaneni TRKC, Konttinen J, Hukka TI, Moilanen A (2016) Influence of torrefaction pretreatment on the pyrolysis of eucalyptus clone: a study on kinetics, reaction mechanism and heat flow. Ind Crop Prod 92:244–254
Sharma P, Diwan PK (2017) Study of thermal decomposition process and the reaction mechanism of eucalyptus wood. Wood Sci Technol 51:1081–1094
Poletto M, Zattera AJ, Santana RMC (2012) Thermal decomposition of wood: kinetics and degradation mechanisms. Bioresour Technol 126:7–12
Junges J, Silvestre WP, Conto DD, Baldasso C, Osório E, Godinho M (2020) Non-isothermal kinetic study of fodder radish seed cake pyrolysis: performance of model-free and model-fitting methods. Braz J Chem Eng 37:139–155
Cao H, Xin Y, Wang D, Yuan Q (2014) Pyrolysis characteristics of cattle manures using a discrete distributed activation energy model. Bioresour Technol 172:219–225
Maia AAD, Morais LC (2016) Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour Technol 204:157–163
Xu Y, Chen B (2013) Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour Technol 146:485–493
Rasool T, Srivastava VC, Khan MNS (2019) Kinetic and thermodynamic analysis of thermal decomposition of deodar (Cedrus deodara) saw dust and rice husk as potential feedstock for pyrolysis. Int J Chem React Eng 17(1):20170184
Ahmad MS, Mehmood MA, Taqvi STH, Elkamel A, Liu CG, Ren X, Rahimuddin SA, Gull M (2017) Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour Technol 245:491–501
Tabal A, Barakat A, Aboulkas A, El harfi K (2021) Pyrolysis of ficus nitida wood: determination of kinetic and thermodynamic parameters. Fuel 283:119253
Xiao H, Jiang K, Chen Y, Lei Z, Chen K, Cheng X, Qi J, Xie J, Huang X, Jiang Y (2020) Kinetics and thermodynamic analysis of recent and ancient buried Phoebe zhennan wood. ACS Omega 5(33):20943–20952
Acknowledgements
The authors are truly grateful to the Department of Chemical Engineering and CIFC IIT-BHU, Varanasi, India for providing essential facilities for completing the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dave, A., Gupta, G.K. & Mondal, M.K. Study on thermal degradation characteristics, kinetics, thermodynamic, and reaction mechanism analysis of Arachis hypogaea shell pyrolysis for its bioenergy potential. Biomass Conv. Bioref. 13, 9289–9304 (2023). https://doi.org/10.1007/s13399-021-01749-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01749-7