Abstract
Background
Gold nanoparticles (AuNPs) have potential for a wide range of applications as therapeutic and diagnostic agents. Since they have a high probability of interacting with human immune cells, cytotoxicity studies must be conducted. The investigation of AuNP/immune cell interaction has mainly focused on macrophages and dendritic cells, along with some other cell lineages. Scarce information is available regarding the effect of AuNPs on mast cells, which are abundant in the skin, mucosa, and perivascular space.
Objective
To examine the uptake of AuNPs by HMC-1 human mast cells and the resulting effect on cell viability, pro-inflammatory mediators production, and proliferation.
Results
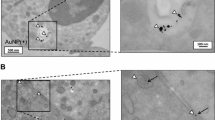
With AuNPs treatment, the viability of HMC-1 cells decreased slightly (never less than 95%) during the first 4 h, but no changes were detected in the proliferation rate at any time. Increasing concentrations of AuNPs produced greater cell granularity (uptake). CLSM images exhibited AuNPs clusters in the cell cytoplasm. TNF-α and ROS production was not stimulated by AuNPs treatment at any concentration/time.
Conclusion
Internalization of AuNPs into HMC-1 cells was demonstrated in an in vitro model, without showing cytotoxic effects or induction of pro-inflammatory mediators at any concentration tested.









Similar content being viewed by others
References
Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE (2009) Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev 61:428–437
Aldossari AA, Shannahan JH, Podila R, Brown JM (2015) Influence of physicochemical properties of silver nanoparticles on mast cell activation and degranulation. Toxicol Vitr 29:195–203
Alsaleh NB, Brown JM (2018) Immune responses to engineered nanomaterials: current understanding and challenges. Curr Opin Toxicol 10:8–14
Ansari SA et al. (2019) Gold nanoparticles synthesized with Smilax glabra rhizome modulates the anti-obesity parameters in high-fat diet and streptozotocin induced obese diabetes rat model. J Photochem Photobiol B Biol 201:111643.
BioLegend (n.d.) 7-AAD Viability Staining Solution. BioLegend. https://www.biolegend.com/en-us/products/7-aad-viability-staining-solution-1649
Boisselier E, Astruc D (2009) Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev 38:1759–1782
Boyd RD, Pichaimuthu SK, Cuenat A (2011) New approach to inter-technique comparisons for nanoparticle size measurements; using atomic force microscopy, nanoparticle tracking analysis and dynamic light scattering. Colloids Surfaces A Physicochem Eng Asp 387:35–42
Campillo-Navarro M et al (2014) Mast cells in lung homeostasis: beyond type I hypersensitivity. Curr Respir Med Rev 10:115–123
Carnovale C, Bryant G, Shukla R, Bansal V (2016) Size, shape and surface chemistry of nano-gold dictate its cellular interactions, uptake and toxicity. Prog Mater Sci 83:152–190
Chen H et al (2018) Gold nanoparticles improve metabolic profile of mice fed a high-fat diet. J Nanobiotechnology 16:1–12
Chithrani BD, Chan WCW (2007) Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett 7:1542–1550
Chithrani BD, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668
Cho WS et al (2009) Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol 236:16–24
Cleyrat C et al (2013) The architectural relationship of components controlling mast cell endocytosis. J Cell Sci 126:4913–4925
Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1:325–327
Cui W et al. (2012) Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomed Nanotechnol Biol Med 8:46–53.
Devika Chithrani B, Ghazani AA, Chan WCW (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6: 662–668.
Dey AK et al. (2021) Impact of Gold Nanoparticles on the Functions of Macrophages and Dendritic Cells. Cells 10.
Dobrovolskaia MA, McNeil SE (2007) Immunological properties of engineered nanomaterials. Nat Nanotechnol 2:469–478
Du S et al. Aggregation and adhesion of gold nanoparticles in phosphate buffered saline. J Nanoparticle Res. 14, (2012).
Duguay BA, Lu L, Arizmendi N, Unsworth LD, Kulka M (2020) The possible uses and challenges of nanomaterials in mast cell research. J Immunol 204:2021–2032
Dykman L, Khlebtsov N (2012) Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev 41:2256–2282
Dykman LA, Khlebtsov NG (2017) Immunological properties of gold nanoparticles. Chem Sci 8:1719–1735
Gallego-Urrea JA, Tuoriniemi J, Hassellöv M (2011) Applications of particle-tracking analysis to the determination of size distributions and concentrations of nanoparticles in environmental, biological and food samples. TrAC—Trends Anal Chem 30:473–483
Khademi S et al (2018) Evaluation of size, morphology, concentration, and surface effect of gold nanoparticles on X-ray attenuation in computed tomography. Phys Medica 45:127–133
Kim JY, Ro JY (2005) Signal pathway of cytokines produced by reactive oxygen species generated from phorbol myristate acetate-stimulated HMC-1 cells. Scand J Immunol 62:25–35
Klippstein R, Fernandez-Montesinos R, M, P, P, A, Pozo D (2010) Silver Nanoparticles Interactions with the Immune System: Implications for Health and Disease. In: Silver Nanoparticles. InTech.
Kumar D, Saini N, Jain N, Sareen R, Pandit V (2013) Gold nanoparticles: an era in bionanotechnology. Expert Opin Drug Deliv 10:397–409
Lebedová J, Hedberg YS, Odnevall Wallinder I, Karlsson HL (2018) Size-dependent genotoxicity of silver, gold and platinum nanoparticles studied using the mini-gel comet assay and micronucleus scoring with flow cytometry. Mutagenesis 33:77–85.
Logozzi M et al. (2019) Human primary macrophages scavenge AuNPs and eliminate it through exosomes. A natural shuttling for nanomaterials. Eur J Pharm Biopharm 137:23–36.
Marquis BJ, McFarland AD, Braun KL, Haynes CL (2008) Dynamic measurement of altered chemical messenger secretion after cellular uptake of nanoparticles using carbon-fiber microelectrode amperometry. Anal Chem 80:3431–3437
Marshall JS (2004) Mast-cell responses to pathogens. Nat Rev Immunol 4:787–799
Mateo D, Morales P, Ávalos A, Haza AI (2014) Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol Mech Methods 24:161–172
Maurer-Jones MA, Lin YS, Haynes CL (2010) Functional assessment of metal oxide nanoparticle toxicity in immune cells. ACS Nano 4:3363–3373
Mukherjee P et al (2005) Antiangiogenic properties of gold nanoparticles. Clin Cancer Res 11:3530–3534
Pissuwan D, Valenzuela SM, Cortie MB (2006) Therapeutic possibilities of plasmonically heated gold nanoparticles. Trends Biotechnol 24:62–67
Prabha S, Arya G, Chandra R, Ahmed B, Nimesh S (2016) Effect of size on biological properties of nanoparticles employed in gene delivery. Artif Cells Nanomed Biotechnol 44:83–91.
Sahay G, Alakhova DY, Kabanov AV (2010) Endocytosis of nanomedicines. J Control Release 145:182–195
Salatin S, Maleki Dizaj S, Yari Khosroushahi A (2015) Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol Int 39:881–890.
Sapsford KE et al (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113:1904–2074
Singh P et al. (2018) Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci 19.
Sundström M et al (2003) Functional and phenotypic studies of two variants of a human mast cell line with a distinct set of mutations in the c-kit proto-oncogene. Immunology 108:89–97
Sundström C et al (2006) Phenotypic characterization of the human mast-cell line HMC-1. Scand J Immunol 39:489–498
Suzuki H, Toyooka T, Ibuki Y (2007) Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ Sci Technol 41:3018–3024
Theoharides TC, Conti P (2004) Mast cells: The JEKYLL and HYDE of tumor growth. Trends Immunol 25:235–241
Thermo Fisher Cientific (n.d.-b) CM-H2DCFDA (General Oxidative Stress Indicator). https://www.thermofisher.com/order/catalog/product/C6827#/C6827
Thermo Fisher Cientific. (n.d.-a). CellTrace TM CFSE Cell Proliferation Kit - For Flow Cytometry. https://www.thermofisher.com/order/catalog/product/C34554
Toma HE, Zamarion VM, Toma SH, Araki K (2010) The coordination chemistry at gold nanoparticles. J Braz Chem Soc 21:1158–1176
Tomic S et al (2014) Size-dependent effects of gold nanoparticles uptake on maturation and antitumor functions of human dendritic cells in vitro. PLoS ONE 9:96584
Trautmann A, Krohne G, Brocker EB, Klein CE (1998) Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol 160:5053–5057
Yamaki K, Yoshino S (2009) Comparison of inhibitory activities of zinc oxide ultrafine and fine particulates on IgE-induced mast cell activation. Biometals 22:1031–1040
Yasinska IM et al (2019) Targeting of basophil and mast cell pro-allergic reactivity using functionalised gold nanoparticles. Front Pharmacol 10:1–7
Youhannayee M et al (2019) Physical characterization and uptake of iron oxide nanoparticles of different prostate cancer cells. J Magn Magn Mater 473:205–214
Zamora-Justo JA et al (2019) Polyethylene glycol-coated gold nanoparticles as DNA and atorvastatin delivery systems and cytotoxicity evaluation. J Nanomater 2019:1–11
Acknowledgements
The authors are grateful to Guillermo Pérez-Dimas and Luis Gilberto Pérez-Blas for technical assistance in cell culture, to Dr. Rommel Chacón-Salinas for providing HMC-1 cells, and to the core faculty of flow cytometry of the ESM-IPN under the direction of Dr. Marycarmen Godínez-Victoria. We greatly appreciate the Hematopathology Lab of the ENCB-IPN, Dr. Elba Reyes-Maldonado, Dr. Ruth Angélica Lezama-Palacios, M.S. Erika Rosales-Cruz, and the CNMN-IPN. RAG-C acknowledges the Consejo Nacional de Ciencia y Tecnología (CONACyT) for the PhD scholarship. OR-C acknowledges the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-20210826) for the funding granted. AM-D acknowledges the Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional (SIP-20196318, SIP-20200845) and the Secretaría de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México (SECTEI/271/2019) for the funding granted.
Author information
Authors and Affiliations
Contributions
Conceptualization: RAG-C, OR-C and AM-D; experimental design: RAG-C, OR-C and RF-M; experimental execution: RAG-C; data acquisition and processing: RAG-C; formal analysis: RAG-C and OR-C; funding acquisition: AM-D and OR-C; project administration: AM-D and OR-C; writing original draft: RAG-C and OR-C; writing review: AM-D, OR-C and RF-M.
Corresponding author
Ethics declarations
Conflict of interest
RAG-C declares that he has not conflict of interest. ORC-C declares that he has not conflict of interest. RF-M declares that he has not conflict of interest. AM-D declares that he has not conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gutiérrez-Calleja, R.A., Rodríguez-Cortés, O., Flores-Mejía, R. et al. Gold nanoparticles: uptake in human mast cells and effect on cell viability, inflammatory mediators, and proliferation. Mol. Cell. Toxicol. 17, 439–452 (2021). https://doi.org/10.1007/s13273-021-00152-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-021-00152-7