Abstract
Background
Recent studies suggest that lymph node ratio (LNR) has significantly better prognostic power than N-status in patients with colorectal cancer, in particular when the number of evaluated lymph nodes (LNs) was insufficient. The aim of this study was to assess the prognostic value of LNR in patients with resected synchronous colorectal liver metastases (SCLMs) and less than 12 examined LNs.
Methods
A prospectively maintained database of patients with resected SCLMs was queried for patients with less than 12 LNs evaluated at the time of surgery. X-tile software was used to determine the LNR cutoff value able to divide the patients in two subgroups with distinct prognosis. Overall survival (OS) and disease-free survival (DFS) rates were compared by log-rank test. A multivariate Cox regression analysis identified independent prognostic factors.
Results
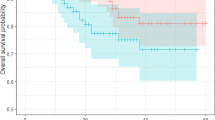
A cutoff LNR value of 0.22 divided patients into Low-LNR group (35 patients) and High-LNR group (36 patients). Both OS and DFS rates were significantly higher in Low-LNR group than those in High-LNR group. Independent predictors of poor OS were High-LNR (HR: 2.841, 95% CI: 1.480–5.453, p value = 0.002), bilobar SCLMs (HR: 2.253, 95% CI: 1.144–4.437, p value = 0.019) and lack of adjuvant chemotherapy (HR: 2.702, 95% CI: 1.448–5.043, p value = 0.002), while the only independent predictor of poor DFS was High-LNR (HR: 2.531, 95% CI: 1.259–5.090, p value = 0.009).
Conclusions
LNR > 0.22 was independently associated with poor OS and DFS in patients with resected SCLMs and less than 12 evaluated LNs.



Similar content being viewed by others
References
Popescu I, Alexandrescu T. Metastatic colorectal cancer: What about the primary? Acta Chir Iugosl 2012; 59: 47–55. [DOI: https://doi.org/10.2298/ACI1202047P]
Wang L-J, Wang H-W, Jin K-M, Li J, Xing B-C. Comparison of sequential, delayed and simultaneous resection strategies for synchronous colorectal liver metastases. BMC Surg 2020; 20: 16. [DOI: https://doi.org/10.1186/s12893-020-0681-7]
Minagawa M, Yamamoto J, Miwa S, Sakamoto Y, Kokudo N, Kosuge T, Miyagawa S, Makuuchi M. Selection Criteria for Simultaneous Resection in Patients With Synchronous Liver Metastasis. ARCH SURG 2006; 141: 7.
Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016; 6: 29765. [DOI: https://doi.org/10.1038/srep29765]
Ozawa T, Ishihara S, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, Hata K, Kawai K, Nozawa H, Kanazawa T, Kazama S, Yamaguchi H, Sunami E, Kitayama J, Watanabe T. Prognostic Significance of the Lymph Node Ratio in Stage IV Colorectal Cancer Patients who have Undergone Curative Resection. Ann Surg Oncol 2015; 22: 1513–1519. [DOI: https://doi.org/10.1245/s10434-014-4184-6]
Ahmad A, Reha J, Saied A, Espat NJ, Somasundar P, Katz SC. Association of primary tumor lymph node ratio with burden of liver metastases and survival in stage IV colorectal cancer. HepatoBiliary Surg Nutr 2017; 6: 154–161. [DOI: https://doi.org/10.21037/hbsn.2016.08.08]
Deng Y, Peng J, Zhao Y, Sui Q, Zhao R, Lu Z, Qiu M, Lin J, Pan Z. Lymph node ratio as a valuable prognostic factor for patients with colorectal liver-only metastasis undergoing curative resection. Cancer Manag Res 2018; 10: 2083–2094. [DOI: https://doi.org/10.2147/CMAR.S169029]
Pei J-P, Zhang C-D, Fan Y-C, Dai D-Q. Comparison of Different Lymph Node Staging Systems in Patients With Resectable Colorectal Cancer. Front Oncol 2019; 8: 671. [DOI: https://doi.org/10.3389/fonc.2018.00671]
Adam R, de Gramont A, Figueras J, Kokudo N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L, Sobrero A, Teh C, Tejpar S, Van Cutsem E, Vauthey J-N, Påhlman L. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat Rev 2015; 41: 729–741. [DOI: https://doi.org/10.1016/j.ctrv.2015.06.006]
Ren J-Q, Liu J-W, Chen Z-T, Liu S-J, Huang S-J, Huang Y, Hong J-S. Prognostic value of the lymph node ratio in stage III colorectal cancer. Chin J Cancer 2012; 31: 241–247. [PMID: 22313594 DOI: https://doi.org/10.5732/cjc.011.10374]
Staging-carcinoma. Available from: https://www.pathologyoutlines.com/topic/colontumorstaging8ed.html. Accessed 4 Apr 2021.
Derwinger K, Gustavsson B. A study of lymph node ratio in stage IV colorectal cancer. World J Surg Oncol 2008; 6: 127. [DOI: https://doi.org/10.1186/1477-7819-6-127]
Jiang K, Zhu Y, Liu Y, Ye Y, Xie Q, Yang X, Wang S. Lymph node ratio as an independent prognostic indicator in stage III colorectal cancer: especially for fewer than 12 lymph nodes examined. Tumour Biol J Int Soc Oncodevelopmental Biol Med 2014; 35: 11685–11690. [PMID: 25139098 DOI: https://doi.org/10.1007/s13277-014-2484-x]
Lu Y-J, Lin P-C, Lin C-C, Wang H-S, Yang S-H, Jiang J-K, Lan Y-T, Lin T-C, Liang W-Y, Chen W-S, Lin J-K, Chang S-C. The impact of the lymph node ratio is greater than traditional lymph node status in stage III colorectal cancer patients. World J Surg 2013; 37: 1927–1933. [PMID: 23609344 DOI: https://doi.org/10.1007/s00268-013-2051-4]
Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol Off J Am Soc Clin Oncol 2005; 23: 8706–8712. [PMID: 16314630 DOI: https://doi.org/10.1200/JCO.2005.02.8852]
Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, Jauch K-W, Kopp R, Pütterich E, Ruppert R, Schuster T, Friess H, Hölzel D. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg 2010; 251: 1070–1078. [PMID: 20485149 DOI: https://doi.org/10.1097/SLA.0b013e3181d7789d]
Nedrebø BS, Søreide K, Nesbakken A, Eriksen MT, Søreide JA, Kørner H. Risk factors associated with poor lymph node harvest after colon cancer surgery in a national cohort. Colorectal Dis 2013; 15: e301–e308. [DOI: https://doi.org/10.1111/codi.12245]
Wong SL, Ji H, Hollenbeck BK, Morris AM, Baser O, Birkmeyer JD. Hospital Lymph Node Examination Rates and Survival After Resection for Colon Cancer. JAMA 2007; 298: 2149. [DOI: https://doi.org/10.1001/jama.298.18.2149]
Reddy SK, Zorzi D, Lum YW, Barbas AS, Pawlik TM, Ribero D, Abdalla EK, Choti MA, Kemp C, Vauthey J-N, Morse MA, White RR, Clary BM. Timing of Multimodality Therapy for Resectable Synchronous Colorectal Liver Metastases: A Retrospective Multi-Institutional Analysis. Ann Surg Oncol 2009; 16: 1809–1819. [DOI: https://doi.org/10.1245/s10434-008-0181-y]
Conci S, Ruzzenente A, Pedrazzani C, Isa G, Turri G, Campagnaro T, Valdegamberi A, Bagante F, Marchitelli I, Guglielmi A. Simultaneous approach for patients with synchronous colon and rectal liver metastases: Impact of site of primary on postoperative and oncological outcomes. Eur J Surg Oncol 2020; 47: S0748798320307861. [DOI: https://doi.org/10.1016/j.ejso.2020.09.015]
Lillemoe HA, Vauthey J-N. Surgical approach to synchronous colorectal liver metastases: staged, combined, or reverse strategy. Hepatobiliary Surg Nutr 2020; 9: 25–34. [DOI: https://doi.org/10.21037/hbsn.2019.05.14]
Ayez N, van der Stok EP, Grünhagen DJ, Rothbarth J, van Meerten E, Eggermont AM, Verhoef C. The use of neo-adjuvant chemotherapy in patients with resectable colorectal liver metastases: Clinical risk score as possible discriminator. Eur J Surg Oncol EJSO 2015; 41: 859–867. [DOI: https://doi.org/10.1016/j.ejso.2015.04.012]
Author information
Authors and Affiliations
Contributions
STA and FMS conception and design of the study, drafting the manuscript; ASD, CAZ, DB, and RTG data acquisition, drafting the manuscript; NOZ statistical analysis, drafting the manuscript; VH and IP analysis and interpretation of data and revising manuscript critically for important intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics Approval
The performance of this retrospective study was approved by the Ethics Committee of Fundeni Clinical Institute, Bucharest, under the number 30769/11.06.2020.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Alexandrescu, S.T., Selaru, F.M., Diaconescu, A.S. et al. Prognostic Value of Lymph Node Ratio in Patients with Resected Synchronous Colorectal Liver Metastases and Less Than 12 Examined Lymph Nodes. J Gastrointest Surg 26, 141–149 (2022). https://doi.org/10.1007/s11605-021-05079-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05079-x