Abstract
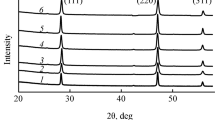
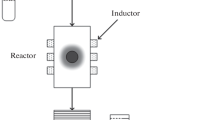
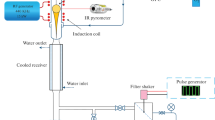
Calcium fluoride powders were prepared by reacting solutions of calcium nitrate (\({{c}_{{{\text{Ca}}{{{({\text{N}}{{{\text{O}}}_{{\text{3}}}})}}_{{\text{2}}}}}}}\) = 0.3846 mol/L) and potassium fluoride (cKF = 0.7692 mol/L) in a microreactor with intensely swirling flows at a reagent flow rate of 2.1–3.2 L/min. Colloidal solutions were thus prepared, whose settling yielded CaF2 powders with a mean size of coherent scattering domains of about 40 nm. The particles had no crystallographic faceting. An increase in reagent flow rate prevented the formation of agglomerates and improved the grain-size uniformity of the powder.



Similar content being viewed by others
REFERENCES
N. P. Yushkin, N. V. Volkova, and G. A. Markova, Optical Fluorite (Nauka, Moscow, 1983) [in Russian].
J. F. Grayson, Micropaleontology 2, 71 (1956).https://doi.org/10.2307/1484493
I. P. Shcherban’, Dokl. AN SSSR, 178, 209 (1968).
P. P. Fedorov, A. A. Luginina, S. V. Kuznetsov, and V. V. Osiko, J. Fluorine Chem. 132, 1012 (2011). https://doi.org/10.1016/j.jfluchem.2011.06.025
F. Wang, X. Fan, D. Pi, and M. Wang, Solid State Commun. 133, 775 (2005).
J. Labeguerie, P. Gredin, M. A. Mortier, et al., Z. Anorg. Allg. Chem. 632, 1538 (2006).
C. Feldmann, M. Roming, and K. Trampert, Small 2, 1248 (2006).
G. Wang, Q. Peng, and Y. Li, J. Am. Chem. Soc. 131, 14200 (2009).
C. Zhang, C. Li, C. Peng, et al., Chem. Eur. J. 16, 5672 (2010).
S. Hou, Y. Zou, X. Yu, et al., CrystEngComm 13, 835 (2011). https://doi.org/10.1039/C0CE00396D
S. V. Kuznetsov, A. S. Nizamutdinov, M. N. Mayakova, et al., J. Fluorine Chem. 222–223, 46 (2019). https://doi.org/10.1016/j.jfluchem.2019.04.010
A. A. Alexandrov, M. N. Mayakova, V. V. Voronov, et al., Condens. Matter Interphases 22, 3 (2020).https://doi.org/10.17308/kcmf.2020.22/2524
S. V. Kuznetsov, A. A. Alexandrov, P. P. Fedorov, Inorg. Mater. 57 (6), 555 (2021).https://doi.org/10.1134/S0020168521060078
A. Bensalah, M. Mortier, G. Patriarche, et al., J. Solid State Chem. 179, 2621 (2006).
J. Sarthou, P. Aballea, G. Patriarche, et al., J. Am. Ceram. Soc. 99 (6), 1992 (2016).
W. Li, H. Huang, B. Mei, et al., Ceram. Int. 46, 19530 (2020). https://doi.org/10.1016/j.ceramint.2020.05.003
Z. Liu, M. Jia, G. Yi, et al., J. Alloys Compd. 646, 760 (2015).
Z. Wan, W. Li, B. Mei, et al., J. Lumin. 223, 117188 (2020).
Yu. Yang, W. Li, B. Mei, et al., J. Lumin. 213, 504 (2019).
Z. Zhou, W. Li, J. Song, et al., J. Eur. Ceram. Soc. 39, 2446 (2019).
S. V. Kuznetsov, I. V. Yarotskya, P. P. Fedorov, et al., Russ. J. Inorg. Chem. 52, 315 (2007). https://doi.org/10.1134/S0036023607030035
P. P. Fedorov, S. V. Kuznetsov, M. N. Mayakova, et al., Russ. J. Inorg. Chem. 56, 1525 (2011). https://doi.org/10.1134/S003602361110007X
Pandurangappa, B. N. Lakshminarappa, and B. M. Nagabhushana, J. Alloys Compd. 489, 592 (2010).
P. P. Fedorov, M. N. Maykova, S. V. Kuznetsov, et al., Russ. J. Inorg. Chem. 61, 1472 (2016). https://doi.org/10.1134/S003602361611005X
P. P. Fedorov, M. N. Mayakova, A. A. Alexandrov, et al., Inorganics 6, 38 (2018). https://doi.org/10.3390/inorganics6020038
P. P. Fedorov and A. A. Alexandrov, J. Fluorine Chem. 227, 109374 (2019). https://doi.org/10.1016/j.jfluchem.2019.109374
V. Yu. Proydakova, A. A. Alexandrov, V. V. Voronov, and P. P. Fedorov, Russ. J. Inorg. Chem. 65, 834 (2020). https://doi.org/10.1134/S0036023620060169
T. Yu. Glazunova, A. I. Boltalin, and P. P. Fedorov, Russ. J. Inorg. Chem. 51, 983 (2006). https://doi.org/10.1134/S0036023606070011
A. E. Glikin, Polymineral-Metasomatic Crystallogenesis (Neva, St. Petersburg, 2004) [in Russian].
K.-T. Vil’ke, Growing Crystals (Nedra, Leningrad, 1977) [in Russian].
I. V. Melikhov, V. F. Komarov, and Yu. A. Kozel, Kolloid. Zhurn. 50, 690 (1988).
Y. Mao, F. Zhang, and S. S. Wong, Adv. Mater. 18, 1895 (2006).
L. Wang, B. Wang, X. Wang, and W. Liu, Tribology Inter. 40, 1179 (2007).
X. Zhang, Z. Quan, J. Yang, et al., Nanotecnology 19, 075603 (2008).
R. Mashiach, H. Weissman, L. Avram, et al., Nature Comm. 12, 229 (2021). https://doi.org/10.1038/s41467-020-20512-6
T. Demkiv, M. Chylii, V. Vistovskyy, et al., J. Lumin. 190, 10 (2017).
V. V. Vistovskyy, A. V. Zhyshkovych, O. O. Halyatkin, et al., J. Appl. Phys. 116, 054308 (2014). https://doi.org/10.1063/1.4892112
S. Kuznetsov, Yu. Ermakova, V. Voronov, et al., J. Mater. Chem. C. 6, 598 (2018). https://doi.org/10.1039/c7tc04913g
O. V. Proskurina, R. S. Abiev, D. P. Danilovich, et al., Chem. Eng. Process: Process Int. 143, 107598 (2019). https://doi.org/10.1016/j.cep.2019.107598
A. V. Zdravkov, Y. S. Kudryashova, and R. S. Abiev, Russ. J. Gen. Chem. 90, 1677 (2020). https://doi.org/10.1134/S1070363220090145
Y. S. Kudryashova, A. V. Zdravkov, V. L. Ugolkov, and R. S. Abiev, Glass Phys. Chem. 46, 335 (2020). https://doi.org/10.1134/S1087659620040082
Y. Albadi, A. A. Sirotkin, V. G. Semenov, R. S. Abiev, and V. I. Popkov, Russ. Chem. Bull. 69, 1290 (2020).
O. V. Proskurina, E. V. Sivtsov, M. O. Enikeeva, et al., Nanosyst.: Phys., Chem., Math. 10, 206 (2019). https://doi.org/10.17586/222080542019102206214
J. Bałdyga and J. R. Bourne, Encyclopedia of Fluid Mechanics, Ed. by N. P. Cheremisinoff, vol. 1 (Gulf Publishing Company, Houston, 1986).
J. Bałdyga and J. R. Bourne, Turbulent Mixing and Chemical Reactions (Wiley, Chichester, 1999).
A. Ghanem, T. Lemenand, D. Della Valle, and H. Peerhossaini, Chem. Eng. Res. Des. 92, 205 (2014).
R. Sh. Abiev, Teor. Found. Chem. Eng. 54, 1131 (2020). https://doi.org/10.1134/S0040579520060019
L. Falk and J.-M. Commenge, Chem. Eng. Sci. 65, 405 (2010).
R. Sh. Abiev, RU Patent No. 2 736 287, Byull. Izobret., No. 32, 2020.
R. Sh. Abiev and A. A. Sirotkin, Fluids 5, 179 (2020). https://doi.org/10.3390/fluids5040179
P. P. Fedorov and V. V. Osiko, Dokl. Phys. 64, 353 (2019). https://doi.org/10.1134/S1028335819090076
R. E. Thoma, Advances in Molten Salt Chemistry, Ed. by J. Braunstein, G. Mamantov, and G. P. Smith (Plenum Press, New York-London, 1975), vol. 3.
W. L. W. Ludekens and A. J. E. Welch, Acta Crystallogr. 5, 841 (1952).
S. V. Kuznetsov, A. A. Ovsyannikova, E. A. Tupitsyna, et al., J. Fluorine Chem. 161, 95 (2014). https://doi.org/10.1016/j.jfluchem.2014.02.011
ACKNOWLEDGMENTS
The facilities of the Shared Facilities Center of the Prokhorov General Physics Institute of the Russian Academy of Sciences and the Shared Facilities Center of the Kurnakov Institute of the Russian Academy of Sciences were used.
Funding
The work was in part supported by the Ministry of Science and Higher Education of the Russian Federation (project no. 0097-2019-0017) and in part supported by the Russian Foundation for Basic Research (project no. 18-29-12050-MK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Abiev, R.S., Zdravkov, A.V., Kudryashova, Y.S. et al. Synthesis of Calcium Fluoride Nanoparticles in a Microreactor with Intensely Swirling Flows. Russ. J. Inorg. Chem. 66, 1047–1052 (2021). https://doi.org/10.1134/S0036023621070020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621070020