Abstract
Urbanization constitutes one of the major transformations of natural habitats, creating new areas characterized by multiple potential wildlife stressors. Birds that live in highly anthropized zones are confronted with physiological and behavioural challenges caused by these stressors. Here, we investigated if several health parameters differed between three subpopulations of tree sparrow nestlings subjected to different levels of anthropogenic pollution, and particularly noise pollution: a quiet rural area, a noisy rural area adjacent to an airport and a heavily urbanized area. We compared body condition, oxidative stress markers and baseline corticosterone levels, expecting urban nestlings to be in overall worse condition as compared to rural (rural and rural airport) birds. In addition, we expected nestlings exposed to aircraft noise to show intermediate stress levels. We found that rural-airport nestlings had the highest levels of antioxidant capacity of plasma and did not differ from rural counterparts in the rest of the parameters. By contrast, urban nestlings were in slightly worse body condition and had lower antioxidant capacity than rural and rural-airport individuals. Our results suggest that aircraft noise does not constitute a significant stressor for nestlings. In contrast, urban conditions constitute a more challenging situation, negatively impacting different physiological systems. Although nestlings seem able to buffer these challenges in the short-term, further research should explore the long-term potential consequences of early exposure to these conditions.
Similar content being viewed by others
Introduction
Urbanization is dramatically transforming natural landscapes and altering ecological processes (Marzluff 2001). This conversion of natural habitats into cities entails several changes for wildlife populations, as some species are unable to cope with this new environment (“urban avoiders”) and others are able to survive and reproduce in the city (“urban exploiters”). Urban areas can provide milder temperatures and higher predictability of food resources (Partecke et al. 2006). However, life in the city is not exempt from costs. Urban areas concentrate a wide range of pollutants derived from human activities such as light (Chepesiuk 2009), chemicals (Brown et al. 2009) and noise (Barber et al. 2010) that can be perceived as stressors. This chronic exposure to stressors can induce changes in glucocorticoid secretion (Partecke et al. 2006; Bonier et al. 2007; Zhang et al. 2011), oxidative stress (Isaksson et al. 2005; Herrera-Dueñas et al. 2014) and behaviour of urban fauna (Lowry et al. 2013).
In particular, noise is a type of pollutant described as any intense sound, which is perceived as unwanted, unpleasant and often stressful (Pepper et al. 2003). Anthropogenic noise is widespread due to urban expansion and amplification of transportation networks (Barber et al. 2010). Anthropogenic noise is ubiquitous, loud and is mainly formed by low frequencies (Francis et al. 2009), representing a challenge for those taxa that depend on acoustic signals for communication, such as birds. Coping with noise may lead to adaptive modifications of bird song characteristics (Bermúdez-Cuamatzin et al. 2011; Slabbekoorn et al. 2003; Nemeth and Brumm 2009), song timing (Fuller et al. 2007), foraging behaviour (Gil et al. 2016; Kight and Swaddle 2011) or habitat use (Halfwerk et al. 2016). In addition, the impairment of parent–offspring communication (Schroeder et al. 2012) may lead to changes in parental provisioning (Leonard and Horn 2012) that may affect growth and body condition of nestlings. These alterations can ultimately affect avian reproductive success and population health (Shannon et al. 2016), although the proximate mechanisms implicated in these effects are still poorly known (Halfwerk et al. 2011).
Perturbations experienced by animals may lead to an increase in the levels of glucocorticoids (mainly corticosterone—henceforth, CORT—in birds), which are steroid hormones responsible for maintaining homeostasis in the organism. In the absence of perturbations, the organism keeps CORT concentrations at basal levels through modulations of the hypothalamus–pituitary–adrenal (HPA) axis (Sapolsky et al. 2000). Chronic exposure to stressors can disrupt the correct functioning of the HPA axis, altering basal CORT levels either by increasing or reducing them (Dickens and Romero 2013; Injaian et al. 2020). Therefore, long-term exposure to situations that may alter HPA responses can result in the impairment of different aspects of animal physiology if habituation is not achieved. Especially in early life stages, prolonged high levels of CORT during development can impair growth, worsen body condition, cause immunosuppression or induce hypersensitivity to stressors at adulthood (Hayward and Wingfield 2004; Sockman and Schwabl 2001; Wingfield et al. 1997). Urban areas are exposed to continuous stressful stimuli that are expected to alter CORT physiology. However, a recent meta-analysis has found no consistent differences between urban and rural birds (Iglesias-Carrasco et al. 2020). Regarding noise pollution specifically, results are also contrasting. Studies using nestling birds (incapable of avoiding noise sources) as focal individuals have found that experimental exposure to traffic noise can lead to both increases and decreases of baseline CORT levels (Injaian et al. 2018b, 2019; Crino et al. 2013; Flores et al. 2019) and no effects at all (Meillère et al. 2015a, b; Angelier et al. 2016). Most correlational studies reported no influence of noise exposure on CORT (Crino et al. 2011; Casasole et al. 2017).
Oxidative stress has also received increasing attention as human activities can alter wildlife redox balance. Oxidative stress results from the imbalance between reactive oxygen and nitrogen species and the antioxidant defences of the organism (formed by enzymatic and non-enzymatic components) in favour of the former. This often results in oxidative damage to essential biomolecules (proteins, lipids, DNA) (Halliwell and Gutteridge 2007), provoking impairment of optimal cellular function and potentially leading to disease and a general loss of fitness (Halliwell and Gutteridge 2007). Higher exposure to reactive oxygen and nitrogen species of external origin (e.g. chemical pollutants from anthropogenic activities) has been proposed as a plausible explanation for the differences in oxidative stress between urban and rural bird populations (Isaksson et al. 2005; Herrera-Dueñas et al. 2014). However, other anthropogenic pollutants such as light and noise can also negatively affect oxidative status. Laboratory studies using rats showed that noise increased oxidative damage and free radical concentration and triggered antioxidant enzymatic responses (Demirel et al. 2009; Said and El-Gohary 2016). In nestling birds, experimental studies have reported increases in oxidative stress levels (Injaian et al. 2018a) and upregulations of different components of the antioxidant machinery (Flores et al. 2019). Interestingly, recent studies have reported that nestlings in noisy environments possess shorter telomeres (Meillère et al. 2015a, b; Grunst et al. 2020), a pattern that may be proximally linked to oxidative stress and glucocorticoids (Haussmann et al. 2011, Monaghan 2014). Therefore, developing under a noisy environment may compromise survival and reproductive success in the future.
Urban areas involve an additive effect of diverse sources of stress, which may result in worse body condition or smaller-sized individuals in comparison to those living in the countryside (Liker et al. 2008; Herrera-Dueñas et al. 2017). Chronic disruption of stress physiology system is hypothesized to have detrimental effects in growth and developing systems. In addition, type and quality of food also play an important role, especially in the case of nestlings, which diet requirements are usually more specific than of the adults (e.g. insectivorous-based diet). Despite its higher predictability and abundance, urban food may not be suitable for nestlings, and high-quality food can be scarcer in cities (e.g. invertebrate prey) (Meillère et al. 2017). In the case of the specific effect of noise, it is expected to alter parental provisioning behaviour, interfering with parent–offspring communication, ultimately affecting nestling body condition and growth. This negative influence of noise on body condition has been supported by some empirical studies (Injaian et al. 2018a, b; Brischioux et al. 2017) but not by others (Flores et al. 2019; Angelier et al. 2016; Injaian et al. 2019).
Although road traffic noise is probably the most prevalent source of noise of human origin, airports represent another interesting scenario to address the impact of noise pollution on wildlife. Since aircraft noise is one of the loudest and most disturbing sounds experienced by humans (Pepper et al. 2003), with aircrafts reaching peaks of > 110 dB, wildlife living nearby airports could also be suffering from the exposure to chronic bursts of aircraft noise (Wolfenden et al. 2019). Empirical work in rats and humans has shown that aircraft noise is capable of raising oxidative stress levels (Kröller-Schön et al. 2018), molecules related to stress (e.g. cortisol, catecholamines) or even alter behaviour and cell morphology (Di et al. 2011). In birds, studies have found that birds can modify their behaviour by advancing dawn chorus (Gil et al. 2015), changing their song and time budgets (Sierro et al. 2017) or increasing vigilance and reducing feeding time (Gil et al. 2016). Nestlings of altricial species serve as an ideal model to study physiological and morphological effects of noise because they cannot avoid the source of disturbance, and studies have been focusing in them lately. However, whether airport noise affects the physiological state of developing nestlings has not been addressed to date.
In the present work, we examined the variation of several health parameters across three populations of tree sparrow (Passer montanus, L.) nestlings. These populations are located at three different areas subjected to contrasting levels and types of anthropogenic pollution (a rural, a rural-airport and an urban area). Our objective was to know if body condition, antioxidant parameters (total glutathione, antioxidant capacity of plasma), oxidative damage (reactive oxygen metabolites) and baseline plasma CORT differed between these three locations. We were particularly interested in the effects of the rural-airport area, which shared a highly similar landscape with the rural area but with the particularity of being adjacent to a flight runway. Our aim was to test if these parameters revealed a negative impact of growing close to an airport area given the detrimental effects of noise reported in other studies in free-living birds. We expected urban nestlings to show overall worse body condition and higher physiological stress (both oxidative stress and basal CORT) in comparison with rural and rural-airport areas due to the co-occurrence of different sources of anthropogenic pollution (air, acoustic and light pollution) in the city.
Material and methods
Study species and site
The Eurasian tree sparrow is a sedentary rural species inhabiting riverine scrublands, farmlands and suburban areas. This species raises one to three broods per breeding season, laying the first clutch in April–May, the second one by the end of May–start of June and the last one in July–August. The modal number of eggs per clutch is 5 (García-Navas 2016). Parents feed their nestlings with Lepidoptera larvae during spring whereas in summer they switch to grasshoppers and other insects (Veiga 1990). Despite being a predominantly rural sparrow in Spain, it also breeds in large parks in cities (García-Navas 2016), usually occupying artificial nest-boxes, which is the case of our three study populations.
We conducted this study during the breeding season of 2017 (from May to June), in three different locations: (1) an urban area located in the campus of the Complutense University of Madrid (i.e."urban area"), (2) a riverine rural area adjacent to the runway of the Madrid-Barajas airport (i.e. “rural-airport area”) and (3) another location in the same riverine rural area, but 13 km apart from the airport (i.e. “rural area”). The urban area is located 14 km apart from the rural-airport area and 23 km from the rural area. The urban area consisted on green areas segregated by roads, sidewalks and campus buildings. The rural-airport and the rural areas share a similar habitat: a riparian zone around the Jarama River formed by a gallery forest and surrounded by a mixture of pastureland, crops and wasteland. Satellite images (Fig. 1S) and maps from the Information System of Land Uses of Spain (SIOSE) can be found in the Supplementary Information archive (Fig. 2S, Table 1S). Beyond minor variations in land uses, a main difference between the rural-airport and the rural area is the noise level to which they are exposed due to the presence of the airport in the former. The rural-airport area is subjected to Lden values of 70–75 dB(A) whereas the rural area is subjected to 50–55 dB(A) (noise map available at www.aena-aeropuertos.es; see Gil et al. 2015). Thus, these data show that the rural-airport area suffers much higher noise levels than the rural area. The urban area is characterized by a very high traffic volume which translates into both high air and noise pollution (Lden values vary between 60 and 75 Db(A), data from Mapa Estratégico de Ruido 2016, Ayuntamiento de Madrid) and constant influx of people.
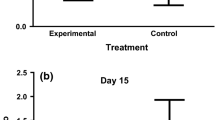
Differences among rural, rural-airport and urban tree sparrow nestlings in body condition (i.e. residuals of weight after controlling for wing length and sex), oxidative damage (plasma ROMs levels), antioxidant capacity (plasma OXY levels), GSH levels in erythrocytes and CORT levels in plasma. Data are reported for first a–e and second broods f–j. Sample size (number of nest boxes) for each habitat is the following for each panel: a 15 and 17, b 14 and 17, c 17 and 14, d 15 and 13, e 14 and 13 for rural and rural-airport areas respectively, and f 10, 11 and 16; g 10, 10 and 10; h 10, 10 and 16; i 10, 10 and 12 and j 10, 10 and 15 for rural, rural-airport and urban areas respectively. Data for urban birds were only available for second broods. Black dots and whiskers indicate mean ± SD
Air pollution data for the three study areas could not be obtained from a single common source. Thus, for the urban area, we obtained air pollution data from a measuring station belonging to the Air Quality Service of the Madrid City Council (the year 2017) located 2 km from our study area. In the case of the rural-airport and rural area, data could only be obtained from the Air Quality Network of the Community of Madrid (the year 2017). Due to different sources of air quality data used for each area, only two indicators are reported for all our study areas: nitrogen oxides (NOx) and nanoparticles (PM2,5, PM10). Both are common pollutants found in cities generated by traffic, thus being potentially associated with exposure to oxidative stress (Isaksson 2015). Levels of NO2 in the rural and rural-airport area reached 17 μg/m3 whereas this compound reached 34 μg/m3 in the urban area, which is close to the limit value determined by current Spanish laws (RD 102/2011). PM2,5 concentration in the rural-airport and rural area was 7 μg/m3 while it reached 8 μg/m3 in the urban area.
Monitoring and sampling protocol
Nest-boxes were regularly examined around the two peaks of breeding (May for the first broods and June for the second broods). When we found eggs in the nest-boxes, we returned 5–7 days later to check whether hatching had occurred and to estimate nestling age. Entire broods were sampled when nestlings reached 11–13 days old, just before fledging.
To sample the birds, after arriving at a nest-box, we quickly descended it with the aid of a pole and took blood samples (150 µl) from all nestlings from the jugular vein using heparinized syringes. To control the effect of handling on CORT levels, we measured the time we took from disturbance to the sampling of each nestling. Blood samples were kept cold until arriving at the lab within 6 h. Blood samples were centrifuged (10,000 rpm, 10 min), and fractions separated and stored at −80 ºC until analysis. We recorded nestling wing length (measured with an end-stop ruler, accuracy = 1 mm) and body weight (measured with a Pesola spring-balance, accuracy = 0.1 g, Switzerland). Unfortunately, we were unable to collect data from the first broods in the urban area. Therefore, our sample is limited to first broods of rural and rural-airport areas and second broods from rural, urban and rural-airport areas. Parent birds were not individually marked in this study population, which prevented us for verifying that breeding pairs were the same in first and second broods of the rural and rural-airport areas. In the case of the rural and rural-airport areas, the number of clutches (and nestlings) from first broods were 13 (47 nestlings) and 10 (36 nestlings) respectively, and 10 (46 nestlings) and 11 (46 nestlings) from the second broods. Lastly, in the urban area, the number of clutches summed up to 16 (51 nestlings). We were not able to collect enough blood to analyse all variables from some nestlings. For this reason, the sample size varies among the different assays.
Oxidative stress assays
Oxidative stress was evaluated using one biomarker of oxidative damage in plasma (reactive oxygen metabolites, ROMs) and two biomarkers of antioxidant defences: antioxidant capacity of plasma (OXY) and total glutathione (GSH) levels in erythrocytes. ROMs, as quantified by the d-ROMs Assay kit (Diacron, Grosetto, Italy), are mostly composed of lipid hydroperoxides, which have been extensively used as biomarkers of oxidative damage (Costantini 2016). Details of the procedure have been described elsewhere (Pérez-Rodríguez et al. 2015). Briefly, we diluted 15 µl of sample on 200 µl solution containing 0.01 M acetic acid/sodium acetate buffer (pH 4.8) and N,N-diethyl-p-phenylenediamine as chromogen. After incubating for 75 min at 37 ºC, absorbance was read with a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments) at 546 nm. The concentration of ROMs (expressed as mg of H2O2 per dL of plasma) was calculated by comparing the absorbance of samples with a calibration standard supplied with the kit. Repeatability of ROMs analyses (after Lessells and Boag 1987), assayed in a random set of samples assayed in duplicate, was 0.92 (F1,10 = 24.4, p < 0.001), and inter-plate CV was 5.1%. The distribution of samples from the three study sites was balanced among assay plates.
The antioxidant capacity of plasma samples was evaluated using the OXY-adsorbent Assay (Diacron, Grosetto, Italy). Five microliters of each plasma sample was diluted in 500 μl distilled water. A 200-μl aliquot of HOCl solution was incubated with 10 μl of the diluted plasma samples for 10 min at 37 °C. The same relative volumes were used for the reference standard supplied with the kit and blank (i.e. water). An alkyl-substituted aromatic amine solubilized in the chromogen is oxidized by the residual HOCl and transformed into a pink derivative. The intensity of the coloured complex, which is inversely related to the antioxidant power, was measured with the same microplate reader mentioned above, at 546 nm. Measurements are expressed as µmol of HOCl neutralized. Repeatability of OXY analyses, assayed in a random set of samples assayed in duplicate, was 0.85 (F1,10 = 13.06, p < 0.001).
GSH is a tripeptide thiol functioning in the protection of cells against free radicals, and it is often considered as one of the most important intracellular antioxidants (Wu et al. 2004). A detailed procedure is described in López-Arrabé et al. (2014). Erythrocytes were diluted (1:10 w/v) and homogenized in PBS-EDTA buffer. Then, GSH was extracted using trichloroacetic acid, and its concentration was quantified in a kinetic reaction involving the NADPH and GSH reductase using the same microplate reader mentioned above. Results are expressed as µmol/g of erythrocytes. Repeatability of GSH analyses, assayed in a random set of samples assayed in duplicate, was 0.89 (F1,54 = 17.6, p < 0.001).
CORT assays
Plasma CORT levels were measured in duplicate for each sample using a specific ELISA and following the manufacturer’s protocols (DRG International, Inc., USA), (see Gil et al. 2019 for further details). The intra-assay coefficient variation as estimated from duplicates was 19.1%, and the inter-assay variation calculated from aliquots ran in each plate was 16.1%. Sample dilutions showed good parallelism with the standard curve. We used the mean value of the duplicates as the response variable for statistical analyses.
Molecular sexing
Previous studies have shown that oxidative stress parameters and CORT-mediated stress responses can differ between male and female birds (e.g. López-Arrabé et al. 2016; Isaksson 2013; Gil et al. 2019). For this reason, when running the GSH analyses, an aliquot of the erythrocyte fraction (~ 20 µl) was added to 250 µl of ethanol absolute in Eppendorf tubes, and stored at 4 °C for molecular sexing. DNA was extracted from these samples using a Chelex (BioRad) extraction method and diluted to a working DNA concentration of 25 ng/μl. This solution was used in a polymerase chain reaction (PCR; using the primers P2 and P8) to amplify a part of the CHD-W gene in females and the CHD-Z gene in both sexes (Griffiths et al. 1998). PCR products were electrophoresed for 60–90 min at 100 V in 2% agarose gels stained with SYBR safe (Invitrogen, Carlsbad, CA) and were visualized under UV light, where one band was scored as male and two bands as female.
Statistical analyses
All analyses were conducted in the R language v. 3.6.1 (R Core Team 2018). Since first clutches from the urban area were not available, we divided the analysis into two separate sets: in the first set, we compared the two broods from the rural-airport and the rural area, whereas in the second set, we compared second broods from rural-airport, rural and the urban area. Haemolysed samples were removed from the analysis of oxidative stress biomarkers, as this is known to affect their values (López-Arrabé et al. 2015; the authors, unpub. data).
We built linear mixed models with the lme4 package (Bates et al. 2015) using restricted maximum likelihood (REML) and an unstructured covariance matrix for the random effect. Models were built for the following response variables: nestling weight, ROMs, OXY, GSH and CORT. We included sex, brood number (first or second) and area as categorical factors in the first set of the analysis. In the case of the second set, we constructed the same models, with the exception that brood number was not considered (as only second broods were included here). Models with weight as response variable also included wing length as a covariate to control for differences in structural development. For this reason, the results of these models are interpreted as “condition” (i.e. size-corrected body weight) instead of as weight. In the case of CORT models, we also added handling time as an additional predictor (Romero and Romero 2002). We tested for the interaction effect between area and brood in the models from the first set analysis, but it never reached significance, being therefore removed from all models presented here. In all linear mixed models, the identity of the nest was included as a random factor. Post hoc comparisons were performed using the Tukey tests. We checked the validity of the models by inspecting residual normality (Shapiro-Wilks’ tests), homogeneity and homoscedasticity.
Results
First and second broods of rural and rural-airport areas
We found no difference in nestling body condition between rural and rural-airport areas (Table 1; Fig. 1a). Nestlings from second broods were in poorer condition than nestlings from first broods, irrespective of the area (Table 1).
ROMs levels in plasma did not differ between rural and rural-airport areas (Table 1; Fig. 1b) but increased from first to second broods (Table 1). By contrast, OXY levels in plasma were higher in the rural-airport area than in the rural area (Table 1; Fig. 1c) while the rest of explanatory variables showed no effect (Table 1). GSH concentration in erythrocytes did not differ between areas (Table 1; Fig. 1d) but was lower in the second broods compared to the first ones (Table 1).
After controlling for handling time, we found no differences in CORT between rural and rural-airport nestlings (Table 1; Fig. 1e). Models reported an overall decrease in CORT from first to second broods (Table 1).
Second broods of rural, rural-airport and urban areas
Nestling body condition was affected by the study area (Table 2; Fig. 1f), although post hoc comparisons revealed only marginal differences between the urban and the other two areas: urban nestlings tended to be on poorer condition than nestlings growing in the rural (estimate ± SE: −1.92 ± 0.89, p = 0.08) and rural-airport area (−1.95 ± 0.07, p = 0.07). Nestlings from the rural and the rural-airport area did not differ in body condition (0.03 ± 0.97, p = 0.99).
In the case of oxidative stress, ROMs levels showed no differences among areas (Table 2; Fig. 1g). By contrast, OXY levels of plasma differed among areas (Table 2). The higher OXY levels were detected in the rural-airport population, and the lowest in the urban area (Fig. 1h). Post hoc tests revealed significant differences between the urban and the rural area (estimate ± SE: −22.60 ± 7.40, p = 0.006), and between the urban and the rural-airport area (−35.51 ± 7.55, p < 0.001), but not between the rural-airport and the rural area (12.91 ± 0.20, p = 0.20). GSH levels in erythrocytes did not differ among areas (Table 2; Fig. 1i).
Finally, after controlling for sampling time, we did not detect differences in CORT among study areas in second broods (Table 2; Fig. 1j).
Discussion
Perturbations originated from humans are expanding rapidly, exposing wildlife to new and pervasive stressors that may compromise or entail costs at individual and population level. In this study, we investigated how different markers of physiological stress and body condition of passerine nestlings varied among three different locations with contrasted levels of noise and air pollution: a rural area, a rural area close to an airport and an urban area. This is to our knowledge the first study to compare the physiological state of developing birds close to an airport. We only found differences among areas in plasma OXY levels, which showed the highest values in the rural-airport area and the lowest in the urban area. Body condition tended to be worse in urban tree sparrows as compared to those from rural and rural-airport areas, and only in second broods. The rest of variables studied (ROMs, GSH and CORT) did not differ among areas, but differed between first and second broods, a pattern that was also present for body condition.
We found a slight effect of the study area on nestling body condition, which tended to be worse in urban nestlings. This trend appeared only in second broods (no data were available from first broods of the urban area). It has been extensively reported that birds dwelling in urban landscapes are often in worse condition, being smaller and leaner than their conspecifics in rural environments (Liker et al. 2008; Meillère et al. 2015a, b; Seress et al. 2020). This suggests that the urban environment imposes constraints on development. Tree sparrow nestlings have an invertebrate-based diet (Veiga 1990). Reduced availability of invertebrates in urban areas (McIntyre 2000) could be one possible explanation to the reduced body condition found in urban tree sparrows. Nevertheless, our correlative approach does not allow us to exclude the synergic effects of other urban features such as air or noise pollution (among others) that could impair urban tree sparrow development.
Surprisingly, nestlings developing next to the airport runway did not differ in body condition from their rural counterparts. Studies about the effects of noise on morphology had yielded contrasted results, including negative effects (Injaian et al. 2018a, b; Potvin and Macdougal-Shackleton 2015), positive associations (Crino et al. 2011, 2013) or even no effects, either by correlational (Crino et al. 2011; Grunst et al. 2020; Raap et al. 2017) or by experimental approaches (Brischioux et al. 2017; Flores et al. 2019; Injaian et al. 2019; Angelier et al. 2016; Meillère et al. 2015a, b). In our study, the rural and rural-airport areas share a very similar habitat, so we expected potential differences in body condition to be driven by the proximity to the airport given that noise can alter both adult (Halfwerk et al. 2011; Francis et al. 2013) and nestling’s behaviour (Leonard and Horn 2012). The fact that we found no difference between rural and rural-airport nestlings suggests that tree sparrow parents are able to fulfil their nestling requirements despite noise. It is possible that previous studies finding effects of noise on body condition may have dealt with stronger or less predictable noise levels. Predictable sources of noise may be less disruptive than unpredictable ones (e.g. road traffic) (Injaian et al. 2018a). Although noise pollution produced by airport activities can reach very high levels (exceeding 70 dB and frequently reaching > 110 dB), it typically increases gradually in strength, and this pattern may be less noxious than unpredictable traffic noise. Besides, noise returns to very low levels when flights have passed, thus allowing intermittent and approximately fixed periods of noise and silence due to the tight schedules of take-offs in large airports. Habituation to chronic stressors has been previously reported in different species (Romero and Wikelski 2002), and it would be plausible that both adult and nestling tree sparrows living next to the airport had become familiar with aircraft noise.
Because of being exposed to anthropogenic stressors, we expected urban and rural-airport tree sparrows to show higher levels of oxidative stress than rural ones. Contrary to our expectations, we did not detect differences among areas in oxidative damage, measured as plasma ROM levels. Erythrocyte levels of GSH, a main endogenous intracellular antioxidant, did not differ among areas either, which is consistent with results reported for great tits (Parus major) from urban and rural areas (Isakson et al. 2005). However, the ratio of oxidized and reduced glutathione (GSSG:GSH) in that study revealed that urban great tits were suffering from higher oxidative stress. Unfortunately, we did not measure this ratio, and we cannot compare our results with these. However, we found that OXY levels were higher in rural-airport tree sparrow nestlings and markedly lower in those from the urban area. OXY captures the pooled antioxidant effect of different non-enzymatic compounds (Costantini 2009) which are mostly obtained from the diet (e.g. flavonoids, carotenoids, vitamins). An elevated antioxidant capacity in the rural-airport area could be interpreted as a surplus of antioxidants derived from a diet rich in these compounds or as an upregulation of defences—due to a release from body stores, for instance—to counteract an oxidative challenge. With the available data, we cannot provide clear support for any of these scenarios. Habitats in the rural and rural-airport area were similar: a riparian area formed by a gallery forest and surrounded by a mixture of pastureland, crops and wasteland. However, we cannot discard the possibility that small differences in structure between these areas (Table 1S) may have provided rural-airport parents access to different prey types that ultimately bolstered the antioxidant capacity of their offspring. Regarding the second possible scenario, the lack of differences in ROMs suggests that if continuous exposure to aircraft noise did constitute an oxidative challenge (which would be in agreement with our initial hypothesis), nestlings elicited an effective response that maintained the redox state under control. Previous studies on nestling birds have found that exposure to noise can lead to an increase of oxidative stress (Injaian et al. 2018a; Raap et al. 2017). However, our results are rather in line with those reported by Casasole et al. (2017), where oxidative stress variables did not show any association with noise amplitude. In second broods, we found markedly lower OXY levels in urban nestlings as compared to rural and rural-airport ones. This is consistent with previous studies in adults of other sparrow species, which have shown that individuals living in rural habitats possess higher values of antioxidant capacity than those living in cities (Herrera-Dueñas et al. 2014, 2017). This may be explained by the lower quantity (see our results for nestling body condition) and antioxidant content of the preys delivered by parents in the urban habitat (Isaksson and Anderssson 2007). An alternative, but not mutually exclusive explanation, is that the combined effect of the different stressors that characterize the city (particularly noise and air pollution) constituted an oxidative challenge that depleted a significant amount of the bioavailable non-enzymatic antioxidants of nestlings. This sets a challenging scenario for urban species or individuals, which may suffer from more diseases, increased mortality or lowered fitness (Isaksson 2015).
Lastly, we did not find differences in basal CORT levels among areas. There is no consensus about how basal CORT levels should vary according to chronic stress (Dickens and Romero 2013), and either enhanced or attenuated basal CORT levels could be expected when exposed to chronic stressors through facilitation or acclimation by the HPA axis (Romero 2004). The complexity of the HPA axis is revealed by the variety of results that can be found in relation to anthropogenic disturbances. While some detected differences between urban and rural populations in baseline CORT levels (Bonier et al. 2007; Zhang et al. 2011), others did not (Partecke et al. 2006; Fokidis et al. 2009), suggesting that these results can depend on the considered species and the characteristics of each urban area. In the case of noise, whereas some studies revealed that traffic noise could elicit an increment or descend in baseline CORT levels (Crino et al. 2011; Kleist et al. 2018, respectively), others detected no change in CORT levels in experimental (Injaian et al. 2019; Angelier et al. 2016) and correlational (Casasole et al. 2017) set ups. The fact that rural-airport nestlings exhibited an overall good body condition and oxidative status may indicate that they do not perceive aircraft noise as a stressor, or that tree sparrow nestlings are habituated to this stressor (Dickens and Romero 2013; Injaian et al. 2020).
Beyond the comparisons among areas, we found that nestlings from second broods in both populations were in worse body condition and showed higher oxidative damage than nestlings from first broods. Seasonal changes in dietary antioxidants and food availability (Arnold et al. 2010), as well as the harshening of environmental conditions, could lead to increases in oxidative damage. In the case of GSH, it is likely that a protein-impoverishment of the diet in second broods could lead to a reduction of GSH concentration, given that synthesis of GSH is protein-dependent and amino acids such as cysteine and methionine are key to its production (Isaksson 2013). Baseline CORT was lower in second broods, suggesting costs associated with the activation of the stress response (Jenni-Eiermann et al. 2008), as shown for other bird species (Blas et al. 2007). In another passerine species, a previous study has found lower basal and response adult CORT levels with advancing date (Romero and Remage-Healey 2000). These data may indicate that advantages derived from higher CORT secretion are checked by survival costs, and these are expected to be higher for second than for first broods.
Finally, it should be noted that the correlational approach of this study claims for some caution regarding the causal inference of our results. Also, our results should be taken with some caution as we compared three different populations reflecting the impact of different anthropogenic factors each: one rural population that was used as a ‘control’ situation; a rural population located in a similar landscape, but exposed to high levels of noise pollution due to the proximity of an airport, and an urban population characterized by a highly anthropic environment, and the pooled effect of air and noise pollution caused by traffic. Despite these limitations, our results add valuable information regarding the impact of these factors on a critical phase of life in birds. Further research, including replicates of the different conditions explored in this study, would allow us to get a better vision about how aircraft noise and urban pollution (both noise and air) could be affecting wildlife. Incorporation of other variables such as fledging success would also help to understand how increasing perturbations might affect the fate of wild populations.
Conclusions
In conclusion, we found that tree sparrow nestlings growing up next to an airport did not differ from their rural counterparts in body condition and oxidative stress. This suggests that tree sparrows dwelling in the surroundings of the airport may be habituated to noise or that they do not perceive aircraft noise as a stressor. In contrast, urban nestlings were in slightly worse body condition and showed lower antioxidant capacity, probably due to shortage in dietary antioxidants or to spending these substances in buffering the oxidative challenge imposed by living in the city. Contrary to our expectations, however, our three different populations of tree sparrow did not differ in GSH, oxidative damage or in baseline CORT. This suggests that if the exposure to noise and air pollution constituted a physiological challenge, they were apparently able to buffer it. Despite its limitations due to the lack of replicates, this study contributes to shed light into the impact of the urban environment and a poorly studied scenario such as airports on physiology. Further research, ideally involving experimental approaches, is sorely needed to evaluate whether the apparent short-term capacity of individuals to buffer the impact of these stressors would result in long-term costs. This would definitely improve our understanding of the effects of anthropogenic sources of stress on wildlife populations.
References
Angelier F, Meillère A, Grace JK et al (2016) No evidence for an effect of traffic noise on the development of the corticosterone stress response in an urban exploiter. Gen Comp Endocrinol 232:43–50. https://doi.org/10.1016/j.ygcen.2015.12.007
Arnold KE, Ramsay SL, Henderson L, Larcombe SD (2010) Seasonal variation in diet quality: antioxidants, invertebrates and blue tits Cyanistes caeruleus. Biol J Linn Soc 99:708–717. https://doi.org/10.1111/j.1095-8312.2010.01377.x
Barber JR, Crooks KR, Fristrup KM (2010) The costs of chronic noise exposure for terrestrial organisms. Trends Ecol Evol 25:180–189. https://doi.org/10.1016/j.tree.2009.08.002
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67. https://doi.org/10.18637/jss.v067.i01
Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM (2011) Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol Lett 7:36–38. https://doi.org/10.1098/rsbl.2010.0437
Blas J, Bortolotti GR, Tella JL et al (2007) Stress response during development predicts fitness in a wild, long lived vertebrate. Proc Natl Acad Sci U S A 104:8880–8884. https://doi.org/10.1073/pnas.0700232104
Bonier F, Martin PR, Sheldon KS et al (2007) Sex-specific consequences of life in the city. Behav Ecol 18:121–129. https://doi.org/10.1093/beheco/arl050
Brischoux F, Meillère A, Dupoué A et al (2017) Traffic noise decreases nestlings’ metabolic rates in an urban exploiter. J Avian Bi 48:905–909. https://doi.org/10.1111/jav.01139
Brown AR, Hosken DJ, Balloux F et al (2009) Genetic variation, inbreeding and chemical exposure - combined effects in wildlife and critical considerations for ecotoxicology. Philos Trans R Soc B Biol Sci 364:3377–3390. https://doi.org/10.1098/rstb.2009.0126
Casasole G, Raap T, Costantini D, AbdElgawad H, Asard H, Pinxten R, Eens M (2017) Neither artificial light at night, anthropogenic noise nor distance from roads are associated with oxidative status of nestlings in an urban population of songbirds. Comp Biochem Physiol -Part A Mol Integr Physiol 210:14–21. https://doi.org/10.1016/j.cbpa.2017.05.003
Chepesiuk R (2009) Missing the dark: health effects of light pollution. Environ Health Perspect 117:20–27. https://doi.org/10.1289/ehp.117-a20
Costantini D (2016) Oxidative stress ecology and the d-ROMs test: facts, misfacts and an appraisal of a decade’s work. Behav Ecol Sociobiol 809–820. https://doi.org/10.1007/s00265-016-2091-5
Costantini D, Verhulst S (2009) Does high antioxidant capacity indicate low oxidative stress? Funct Ecol 23:506–509. https://doi.org/10.1111/j.1365-2435.2009.01546.x
Crino OL, Johnson EE, Blickley JL et al (2013) Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J Exp Biol 216:2055–2062. https://doi.org/10.1242/jeb.081109
Crino OL, Van Oorschot BK, Johnson EE et al (2011) Proximity to a high traffic road: glucocorticoid and life history consequences for nestling white-crowned sparrows. Gen Comp Endocrinol 173:323–332. https://doi.org/10.1016/j.ygcen.2011.06.001
Demirel R, Mollaoǧlu H, Yeşilyurt H et al (2009) Noise induces oxidative stress in rat. Eur J Gen Med 6:20–24. https://doi.org/10.29333/ejgm/82631
Di GQ, Zhou B, Li ZG, Lin QL (2011) Aircraft noise exposure affects rat behavior, plasma norepinephrine levels, and cell morphology of the temporal lobe. J Zhejiang Univ Sci B 12:969–975. https://doi.org/10.1631/jzus.B1000439
Dickens MJ, Romero LM (2013) A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol 191:177–189. https://doi.org/10.1016/j.ygcen.2013.06.014
Flores R, Penna M, Wingfield JC et al (2019) Effects of traffic noise exposure on corticosterone, glutathione and tonic immobility in chicks of a precocial bird. Conserv Physiol 7:1–10. https://doi.org/10.1093/conphys/coz061
Fokidis HB, Orchinik M, Deviche P (2009) Corticosterone and corticosteroid binding globulin in birds: Relation to urbanization in a desert city. Gen Comp Endocrinol 160:259–270. https://doi.org/10.1016/j.ygcen.2008.12.005
Francis CD, Barber JR (2013) A framework for understanding noise impacts on wildlife: an urgent conservation priority in a nutshell. Front Ecol Environ 11:305–313. https://doi.org/10.1890/120183
Francis CD, Ortega CP, Cruz A (2009) Noise pollution changes avian communities and species interactions. Curr Biol 19:1415–1419. https://doi.org/10.1016/j.cub.2009.06.052
Fuller RA, Warren PH, Gaston KJ (2007) Daytime noise predicts nocturnal singing in urban robins. Biol Lett 3:368–370. https://doi.org/10.1098/rsbl.2007.0134
García-Navas V (2016) Gorrión Molinero – Passer montanus. En: Enciclopedia Virtual de los Vertebrados Españoles. Available: http://www.vertebradosibericos.org/aves/pasmon.html (accessed April 2019)
Gil D, Alfonso-Iñiguez S, Pérez-Rodríguez L, Muriel J, Monclús R (2019) Harsh conditions during early development influence telomere length in an altricial passerine: links with oxidative stress and corticosteroids
Gil D, Honarmand M, Pascual J, Pérez-Mena E, Macías Garcia C (2015) Birds living near airports advance their dawn chorus and reduce overlap with aircraft noise. Behav Ecol 26:435–443. https://doi.org/10.1093/beheco/aru207
Gil D, Klett-mingo JI, Pav I (2016) Great tits, Parus major, increase vigilance time and reduce feeding effort during peaks of aircraft noise. Anim Behav 115:29–34. https://doi.org/10.1016/j.anbehav.2016.02.021
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075. https://doi.org/10.1046/j.1365-294x.1998.00389.x
Grunst ML, Grunst AS, Pinxten R, Eens M (2020) Anthropogenic noise is associated with telomere length and carotenoid-based coloration in free-living nestling songbirds. Environ Pollut 260:114032. https://doi.org/10.1016/j.envpol.2020.114032
Halfwerk W, Amsterdam VU, Both C (2016) Behavioral ecology noise affects nest-box choice of 2 competing songbird species, but not their reproduction. Behav Ecol 00:1–9. https://doi.org/10.1093/beheco/arw095
Halfwerk W, Holleman LJM, Lessells CKM, Slabbekoorn H (2011) Negative impact of traffic noise on avian reproductive success. J Appl Ecol 48:210–219. https://doi.org/10.1111/j.1365-2664.2010.01914.x
Halliwel B, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford Press, Oxford
Haussmann MF, Longenecker AS, Marchetto NM et al (2012) Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B Biol Sci 279:1447–1456. https://doi.org/10.1098/rspb.2011.1913
Hayward LS, Wingfield JC (2004) Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol 135:365–371. https://doi.org/10.1016/j.ygcen.2003.11.002
Herrera-Dueñas A, Pineda J, Antonio MT, Aguirre JI (2014) Oxidative stress of house sparrow as bioindicator of urban pollution. Ecol Indic 42:6–9. https://doi.org/10.1016/j.ecolind.2013.08.014
Herrera-Dueñas A, Pineda-Pampliega J, Antonio-García MT, Aguirre JI (2017) The influence of urban environments on oxidative stress balance: a case study on the house sparrow in the Iberian Peninsula. Front Ecol Evol 5:1–10. https://doi.org/10.3389/fevo.2017.00106
Iglesias-Carrasco M, Aich U, Jennions MD, Head ML (2020) Stress in the city: meta-analysis indicates no overall evidence for stress in urban vertebrates: stressful cities: a meta-analysis. Proc R Soc B Biol Sci 287. https://doi.org/10.1098/rspb.2020.1754
Injaian AS, Francis CD, Ouyang JQ, Dominoni DM, Donald JW, Fuxjager MJ, Goymann W, Hau M, Husak JF, Michele A, Kircher BK, Knapp R, Martin LB, Miller ET, Laura A (2020) Baseline and stress-induced corticosterone levels across birds and reptiles do not reflect urbanization levels. Conserv Physiol 8:1–18. https://doi.org/10.1093/conphys/coz110
Injaian AS, Gonzalez-Gomez PL, Taff CC et al (2019) Traffic noise exposure alters nestling physiology and telomere attrition through direct, but not maternal, effects in a free-living bird. Gen Comp Endocrinol 276:14–21. https://doi.org/10.1016/j.ygcen.2019.02.017
Injaian AS, Taff CC, Patricelli GL (2018a) Experimental anthropogenic noise impacts avian parental behaviour, nestling growth and nestling oxidative stress. Anim Behav 136:31–39. https://doi.org/10.1016/j.anbehav.2017.12.003
Injaian AS, Taff CC, Pearson KL, Gin MMY, Patricelli GL, Vitousek MN (2018b) Effects of experimental chronic traffic noise exposure on adult and nestling corticosterone levels, and nestling body condition in a free-living bird. Horm Behav 106:19–22. https://doi.org/10.1016/j.yhbeh.2018.07.012
Isaksson C (2013) Opposing effects on glutathione and reactive oxygen metabolites of sex, habitat, and spring date, but no effect of increased breeding density in great tits (parus major). Ecol Evol 3:2730–2738. https://doi.org/10.1002/ece3.663
Isaksson C (2015) Urbanization, oxidative stress and inflammation: a question of evolving, acclimatizing or coping with urban environmental stress. Funct Ecol 29:913–923. https://doi.org/10.1111/1365-2435.12477
Isaksson C, Andersson S (2007) Carotenoid diet and nestling provisioning in urban and rural great tits Parus major. J Avian Biol 38:564–572. https://doi.org/10.1111/j.2007.0908-8857.04030.x
Isaksson C, Örnborg J, Stephensen E, Andersson S (2005) Plasma glutathione and carotenoid coloration as potential biomarkers of environmental stress in great tits. EcoHealth 2:138–146. https://doi.org/10.1007/s10393-005-3869-5
Jenni-Eiermann S, Glaus E, Grüebler M et al (2008) Glucocorticoid response to food availability in breeding barn swallows (Hirundo rustica). Gen Comp Endocrinol 155:558–565. https://doi.org/10.1016/j.ygcen.2007.08.011
Kleist NJ, Guralnick RP, Cruz A et al (2018) Chronic anthropogenic noise disrupts glucocorticoid signaling and has multiple effects on fitness in an avian community. Proc Natl Acad Sci U S A 115:E648–E657. https://doi.org/10.1073/pnas.1709200115
Kröller-Schön S, Daiber A, Steven S et al (2018) Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur Heart J 39:3528–3539. https://doi.org/10.1093/eurheartj/ehy333
Leonard ML, Horn AG (2012) Ambient noise increases missed detections in nestling birds. Biol Lett 8:530–532. https://doi.org/10.1098/rsbl.2012.0032
Lessells CM, Boag PT (1987) Unrepeatable Repeatabilities: a Common Mistake Auk 104:116–121
Liker A, Papp Z, Bókony V, Lendvai ÁZ (2008) Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. J Anim Ecol 77:789–795. https://doi.org/10.1111/j.1365-2656.2008.01402.x
López-Arrabé J, Cantarero A, Pérez-Rodríguez L et al (2016) Oxidative stress in early life: associations with sex, rearing conditions, and parental physiological traits in nestling pied flycatchers. Physiol Biochem Zool 89:83–92. https://doi.org/10.1086/685476
López-Arrabé J, Cantarero A, Pérez-Rodríguez L, Palma A, Alonso-Alvarez C, González-Braojos S, Moreno J (2015) Nest-dwelling ectoparasites reduce antioxidant defences in females and nestlings of a passerine: a field experiment. Oecologia 179:29–41. https://doi.org/10.1007/s00442-015-3321-7
López-Arrabé J, Cantarero A, Pérez-Rodríguez L, Palma A, Moreno J (2014) Experimental pyrethroid treatment underestimates the effects of ectoparasites in cavity-nesting birds due to toxicity. Ibis (Lond 1859) 156:606–614. https://doi.org/10.1111/ibi.12160
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549. https://doi.org/10.1111/brv.12012
Marzluff JM (2001) Worldwide urbanization and its effects on birds. In: Massa R (ed) Avian Ecology and Conservation in an Urbanizing World, Marzluff, JM, Bowman, R, Donnelly. Kluwer Academic Publishers, Massachusetts, pp 19–47
McIntyre NE (2000) Ecology of urban arthropods: a review and a call to action. Ann Entomol Soc Am 93:825–835. https://doi.org/10.1603/0013-8746(2000)093
Meillère A, Brischoux F, Henry PY et al (2017) Growing in a city: consequences on body size and plumage quality in an urban dweller, the house sparrow (Passer domesticus). Landsc Urban Plan 160:127–138. https://doi.org/10.1016/j.landurbplan.2016.12.014
Meillère A, Brischoux F, Parenteau C, Angelier F (2015a) Influence of urbanization on body size, condition, and physiology in an urban exploiter: a multi-component approach. PLoS ONE 10:1–19. https://doi.org/10.1371/journal.pone.0135685
Meillère A, Brischoux F, Ribout C, Angelier F (2015b) Traffic noise exposure affects telomere length in nestling house sparrows. Biol Lett 11:20150559. https://doi.org/10.1098/rsbl.2015.0559
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217:57–66. https://doi.org/10.1242/jeb.090043
Nemeth E, Brumm H (2009) Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim Behav 78:637–641. https://doi.org/10.1016/j.anbehav.2009.06.016
Partecke J, Schwabl I, Gwinner E (2006) Stress and the city: urbanization and its effects on the stress physiology in European Blackbirds. Ecology 87:1945–1952. https://doi.org/10.1890/0012-9658
Pepper CB, Nascarella MA, Kendall RJ (2003) A review of the effects of aircraft noise on wildlife and humans, current control mechanisms, and the need for further study. Enviromental Manag 32:418–432
Pérez-Rodríguez L, Romero-Haro AA, Sternalski A, Muriel J, Mougeot F, Gil D, Alonso-Alvarez C (2015) Measuring oxidative stress: the confounding effect of lipid concentration in measures of lipid peroxidation. Physiol Biochem Zool 88:345–351
Potvin DA, MacDougall-Shackleton SA (2015) Experimental chronic noise exposure affects adult song in zebra finches. Anim Behav 107:201–207. https://doi.org/10.1016/j.anbehav.2015.06.021
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Kight R, C; P. Swaddle J, (2011) REVIEW AND SYTHESIS: how and why environmental noise impacts animals: an integrative, mechanistic review. Ecol Lett 14:1052–1061. https://doi.org/10.1111/j.1461-0248.2011.01664.x
Raap T, Pinxten R, Casasole G, Dehnhard N, Eens M (2017) Ambient anthropogenic noise but not light is associated with the ecophysiology of free-living songbird nestlings. Sci Rep 7:1–8. https://doi.org/10.1038/s41598-017-02940-5
Romero LM (2004) Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255. https://doi.org/10.1016/j.tree.2004.03.008
Romero LM, Remage-Healey L (2000) Daily and seasonal variation in response to stress in captive starlings (Sturnus vulgaris): corticosterone. Gen Comp Endocrinol 119:52–59. https://doi.org/10.1006/gcen.2000.7491
Romero LM, Romero RC (2002) Corticosterone responses in wild birds: the importance of rapid initial sampling. Condor 104:129. https://doi.org/10.1650/0010-5422(2002)104
Romero LM, Wikelski M (2002) Exposure to tourism reduces stress-induced corticosterone levels in Galápagos marine iguanas. Biol Conserv 108:371–374. https://doi.org/10.1016/S0006-3207(02)00128-3
Said MA, El-Gohary OA (2016) Effects of noise stress on cardiovascular system in adult male albino rat: implication of stress hormones, endothelial dysfunction and oxidative stress. Gen Physiol Biophys 35:371–377. https://doi.org/10.4149/gpb_2016003
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. https://doi.org/10.1210/er.21.1.55
Schroeder J, Nakagawa S, Cleasby IR, Burke T (2012) Passerine birds breeding under chronic noise experience reduced fitness. PLoS ONE 7. https://doi.org/10.1371/journal.pone.0039200
Seress G, Sándor K, Evans KL, Liker A (2020) Food availability limits avian reproduction in the city: an experimental study on great tits Parus major. J Anim Ecol 89:1570–1580. https://doi.org/10.1111/1365-2656.13211
Shannon G, McKenna MF, Angeloni LM, Crooks KR, Fristrup KM, Brown E, Warner KA, Nelson MD, White C, Briggs J, McFarland S, Wittemyer G (2016) A synthesis of two decades of research documenting the effects of noise on wildlife. Biol Rev 91:982–1005. https://doi.org/10.1111/brv.12207
Sierro J, Schloesing E, Pavón I, Gil D (2017) European blackbirds exposed to aircraft noise advance their chorus, modify their song and spend more time singing. Front Ecol Evol 5. https://doi.org/10.3389/fevo.2017.00068
Slabbekoorn H, Peet M, Grier DG (2003) Birds sing at higher pitch in urban noise. Nature 424.
Sockman KW, Schwabl H (2001) Plasma corticosterone in nestling American kestrels: effects of age, handling stress, yolk androgens, and body condition. Gen Comp Endocrinol 122:205–212. https://doi.org/10.1006/gcen.2001.7626
Veiga J (1990) A comparative study of reproductive adaptations in house and tree sparrows. Auk 107:45–59. https://doi.org/10.2307/4087801
Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J (1997) Environmental stress, field endocrinology, and conservation biology. In Behavioral Approaches to Conservation in the Wild, Clemmons J R and Buchholz R (editors), Cambridge, UK: Cambridge University Press, pp. 95–131
Wolfenden AD, Slabbekoorn H, Kluk K, de Kort SR (2019) Aircraft sound exposure leads to song frequency decline and elevated aggression in wild chiffchaffs. J Anim Ecol 88:1720–1731. https://doi.org/10.1111/1365-2656.13059
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492. https://doi.org/10.1093/jn/134.3.489
Zhang S, Lei F, Liu S, Li D, Chem C, Wang P (2011) Variation in baseline corticosterone levels of Tree Sparrow (Passer montanus) populations along an urban gradient in Beijing, China. J Ornithol 152:801–806. https://doi.org/10.1007/s10336-011-0663-8
Acknowledgements
We sincerely thank the contributions of two anonymous reviewers, whose useful and detailed comments greatly improved this manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was supported by a research grant from the Spanish Ministerio de Economía y Competitividad (CGL2014-55577-R) to DG. LP-R was supported by a postdoctoral contract for accessing the Spanish System of Science, Technology, and Innovation (SECTI) from the University of Castilla-La Mancha. IR was supported by a Formación de Personal Universitario (FPU) grant from the Spanish Ministry of Culture and Education. JM was supported by a postdoctoral grant from the Juan de la Cierva Subprogram (FJCI-2017–34109), with the financial sponsorship of the MICINN. JIA work is a contribution to project CGL2017-85637-P of the Spanish Ministry of Economy and Competitiveness.
Author information
Authors and Affiliations
Contributions
Iraida Redondo: formal analyses, data curation, investigation, writing-original draft. Jaime Muriel: data curation, investigation, writing—review and editing. Cristina de Castro: investigation, writing—review & editing. José I. Aguirre: writing—review and editing, resources. Diego Gil: conceptualization, methodology, funding acquisition, supervision, writing—review and editing, project administration. Lorenzo Pérez-Rodríguez: conceptualization, methodology, resources, supervision, writing—review and editing, project administration.
Corresponding author
Ethics declarations
Ethics approval
The welfare of animals used in this study was guaranteed by following all applicable international, national, and/or institutional guidelines and recommendations for care and use of animals. Capture and manipulation of birds were authorized by the Consejería de Medio Ambiente (Comunidad de Madrid, Spain) under license from the Spanish institutional authorities.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Redondo, I., Muriel, J., de Castro Díaz, C. et al. Influence of growing up in the city or near an airport on the physiological stress of tree sparrow nestlings (Passer montanus) . Eur J Wildl Res 67, 68 (2021). https://doi.org/10.1007/s10344-021-01509-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01509-y