Abstract
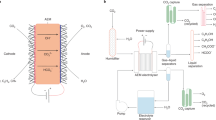
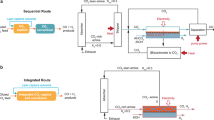
Low-temperature CO2 electrolysis represents a potential enabling process in the production of renewable chemicals and fuels, notably carbon monoxide, formic acid, ethylene and ethanol. Because this technology has progressed rapidly in recent years, a systematic techno-economic assessment has become necessary to evaluate its feasibility as a CO2 utilization approach. Here this work provides a comprehensive techno-economic assessment of four major products and prioritizes the technological development with systematic guidelines to facilitate the market deployment of low-temperature CO2 electrolysis. First, we survey state-of-the-art electrolyser performance and parameterize figures of merit. The analysis shows that production costs of carbon monoxide and formic acid (C1 products) are approaching US$0.44 and 0.59 kg–1, respectively, competitive with conventional processes. In comparison, the production of ethylene and ethanol (C2 products) is not immediately feasible due to their substantially higher costs of US$2.50 and 2.06 kg–1, respectively. We then provide a detailed roadmap to making C2 product production economically viable: an improvement in energetic efficiency to ~50% and a reduction in electricity price to US$0.01 kWh–1. We also propose industrially relevant benchmarks: 5-year stability of electrolyser components and the single-pass conversion of 30 and 15% for C1 and C2 products, respectively. Finally we discuss the economic aspects of two potential strategies to address electrolyte neutralization utilizing either an anion exchange membrane or bipolar membrane.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Code availability
The MATLAB codes for voltammetric profiles are given in Supplementary Data 2.
References
IPCC: Summary for Policymakers. In Special Report on Global Warming of 1.5 °C (eds Masson-Delmotte, V. et al.) (IPCC, WMO, 2018); https://www.ipcc.ch/sr15/chapter/spm/
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Pales, A. F. et al. Exploring Clean Energy Pathways: The Role of CO2 Storage (IEA, 2019); https://www.iea.org/reports/the-role-of-co2-storage/
Roh, K. et al. Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels. Green Chem. 22, 3842–3859 (2020).
Kennedy, G. W. A. Parish Post-Combustion CO2 Capture and Sequestration Demonstration Project Final Technical Report Report DOE-PNPH-03311 (US Department of Energy Office of Scientific and Technical Information, 2020); https://doi.org/10.2172/1608572
Friedmannn, J., Ochu, E. & Brown, J. D. Capturing Investment: Policy Design to Finance CCUS Projects in the US Power Sector (Columbia School of International and Public Affairs, 2020); https://www.energypolicy.columbia.edu/research/report/capturing-investment-policy-design-finance-ccus-projects-us-power-sector
Ekmann, J., Huston, J. & Indrakanti, P. Carbon Capture, Utilization, and Storage: Technology and Policy Status and Opportunities (National Association of Regulatory Utility Commissioners, 2018); https://pubs.naruc.org/pub/09B7EAAA-0189-830A-04AA-A9430F3D1192
Edwards, R. W. & Celia, M. A. Infrastructure to enable deployment of carbon capture, utilization, and storage in the United States. Proc. Natl Acad. Sci. USA 115, E8815–E8824 (2018).
Jhong, H.-R. M., Ma, S. & Kenis, P. J. A. Electrochemical conversion of CO2 to useful chemicals: current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2, 191–199 (2013).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Lu, Q. & Jiao, F. Electrochemical CO2 reduction: electrocatalyst, reaction mechanism, and process engineering. Nano Energy 29, 439–456 (2016).
Martín, A. J., Larrazábal, G. O. & Pérez-Ramírez, J. Towards sustainable fuels and chemicals through the electrochemical reduction of CO2: lessons from water electrolysis. Green Chem. 17, 5114–5130 (2015).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Ma, S. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Alibaba product search: formic acid 85%, category: organic acid, min order: 10 metric tons (Alibaba, 2021); https://www.alibaba.com/trade/search?IndexArea=product_en&SearchText=formic_acid_85%25&c=CID80310&f0=y&moqf=MOQF&moqt=MOQT10%20Metric%20Tons
Ethylene Market Size Worth $186.5 Billion by 2026 (Polaris, 2020); https://www.polarismarketresearch.com/press-releases/ethylene-market
Formic Acid Market Anticipated to Reach Market Value of USD 878.7 Million at a CAGR of 4.94% during 2016 to 2027 (MarketResearchFuture, 2017); https://www.globenewswire.com/news-release/2017/09/08/1116865/0/en/Formic-Acid-Market-Anticipated-to-Reach-Market-Value-of-USD-878-7-Million-at-a-CAGR-of-4-94-during-2016-to-2027.html
Ethanol Market Size Worth Around USD 155.6 Billion by 2030 (PrecedenceResearch, 2021); http://www.globenewswire.com/news-release/2021/01/18/2160198/0/en/Ethanol-Market-Size-Worth-Around-USD-155-6-Billion-by-2030.html#:~:text=The%20global%20ethanol%20market%20size,5.2%25%20from%202021%20to%202030
Global Carbon Monoxide Market 2020–2026, with Breakdown Data of Capacity, Sales, Revenue, Price, Cost and Gross Profit (ReportsNMarkets, 2020); https://www.reportsnmarkets.com/report/Global-Carbon-Monoxide-Market-2020-2026-With-Breakdown-Data-of-Capacity-Sales-Revenue-Price-Cost-and-Gross-Profit–60
Sims, M. US May Ethylene Contracts Settle Up After Six-Month Decline (ICIS, 2020); https://www.icis.com/explore/resources/news/2020/06/02/10514594/us-may-ethylene-contracts-settle-up-after-six-month-decline
Annual Energy Outlook 2021: Table 12: Petroleum and Other Liquids Prices (EIA, 2020); https://www.eia.gov/outlooks/aeo/data/browser/#/?id=12-AEO2021&cases=ref2021&sourcekey=0
Haegel, N. M. et al. Terawatt-scale photovoltaics: trajectories and challenges. Science 356, 141–143 (2017).
Levelized Cost and Levelized Avoided Cost of New Generation Resources (EIA, 2020); https://www.eia.gov/outlooks/aeo/pdf/electricity_generation.pdf
Wiser, R. & Bolinger, M. 2016 Wind Technologies Market Report (US Department of Energy, 2016); https://www.energy.gov/sites/default/files/2017/10/f37/2016_Wind_Technologies_Market_Report_101317.pdf
Baldea, M., Edgar, T. F., Stanley, B. L. & Kiss, A. A. Modular manufacturing processes: status, challenges, and opportunities. AlChE J. 63, 4262–4272 (2017).
Verma, S., Kim, B., Jhong, H. R. M., Ma, S. & Kenis, P. J. A. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 9, 1972–1979 (2016).
Rumayor, M., Dominguez-Ramos, A., Perez, P. & Irabien, A. A techno-economic evaluation approach to the electrochemical reduction of CO2 for formic acid manufacture. J. CO2 Util. 34, 490–499 (2019).
Na, J. et al. General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation. Nat. Commun. 10, 5193 (2019).
Verma, S., Lu, S. & Kenis, P. J. A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption. Nat. Energy 4, 466–474 (2019).
Spurgeon, J. M. & Kumar, B. A comparative technoeconomic analysis of pathways for commercial electrochemical CO2 reduction to liquid products. Energy Environ. Sci. 11, 1536–1551 (2018).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Orella, M. J., Brown, S. M., Leonard, M. E., Román-Leshkov, Y. & Brushett, F. R. A general technoeconomic model for evaluating emerging electrolytic processes. Energy Technol. 8, 1900994 (2019).
Wang, N. et al. Hydration-effect-promoting Ni–Fe oxyhydroxide catalysts for neutral water oxidation. Adv. Mater. 32, 1906806 (2020).
Smith, A. M., Trotochaud, L., Burke, M. S. & Boettcher, S. W. Contributions to activity enhancement via Fe incorporation in Ni-(oxy)hydroxide/borate catalysts for near-neutral pH oxygen evolution. Chem. Commun. 51, 5261–5263 (2015).
Salvatore, D. A. et al. Designing anion exchange membranes for CO2 electrolysers. Nat. Energy 6, 339–348 (2021).
Houache, M. S. E. et al. Selective electrooxidation of glycerol to formic acid over carbon supported Ni1–xMx (M = Bi, Pd, and Au) nanocatalysts and coelectrolysis of CO2. ACS Appl. Energy Mater. 3, 8725–8738 (2020).
Landress, L. Outlook ‘19: US Glycerine Markets Mixed Amid Uncertainty (ICIS, 2019); https://www.icis.com/explore/resources/news/2019/01/07/10301259/outlook-19-us-glycerine-markets-mixed-amid-uncertainty/
The SunShot 2030 Goals: 3¢ Per Kilowatt Hour for PV and 5¢ Per Kilowatt Hour for Dispatchable CSP (US Department of Energy, 2017); https://www.energy.gov/sites/prod/files/2020/09/f79/SunShot%202030%20White%20Paper.pdf
Edwards, J. P. et al. Efficient electrocatalytic conversion of carbon dioxide in a low-resistance pressurized alkaline electrolyzer. Appl. Energy 261, 114305 (2020).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Endrődi, B. et al. High carbonate ion conductance of a robust PiperION membrane allows industrial current density and conversion in a zero-gap carbon dioxide electrolyzer cell. Energy Environ. Sci. 13, 4098–4105 (2020).
Electricity Generation in Germany in January 2020 (Energy-Charts, 2020); https://energy-charts.info/charts/power/chart.htm?l=en&c=DE
Küngas, R. et al. Progress in SOEC development activities at Haldor Topsøe. ECS Trans. 91, 215–223 (2019).
The Tax Credit for Carbon Sequestration (Section 45Q) (Congressional Research Service, 2020); https://fas.org/sgp/crs/misc/IF11455.pdf
FY 2018 Progress Report for the DOE Hydrogen and Fuel Cells Program (US Department of Energy, 2019); https://www.nrel.gov/docs/fy19osti/73353.pdf
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Li, T. et al. Electrolytic conversion of bicarbonate into CO in a flow cell. Joule 3, 1487–1497 (2019).
Salvatore, D. & Berlinguette, C. P. Voltage matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 5, 215–220 (2020).
Sun, C. N. et al. Probing electrode losses in all-vanadium redox flow batteries with impedance spectroscopy. ECS Electrochem. Lett. 2, 2013–2015 (2013).
Heinzmann, M., Weber, A. & Ivers-Tiffée, E. Advanced impedance study of polymer electrolyte membrane single cells by means of distribution of relaxation times. J. Power Sources 402, 24–33 (2018).
Xu, Q. et al. Integrated reference electrodes in anion-exchange-membrane electrolyzers: impact of stainless-steel gas-diffusion layers and internal mechanical pressure. ACS Energy Lett. 6, 305–312 (2020).
Nwabara, U. O. et al. Toward accelerated durability testing protocols for CO2 electrolysis. J. Mater. Chem. A 8, 22557–22571 (2020).
He, M. et al. Oxygen induced promotion of electrochemical reduction of CO2 via co-electrolysis. Nat. Commun. 11, 3844 (2020).
Chae, S. Y. et al. A perspective on practical solar to carbon monoxide production devices with economic evaluation. Sustain. Energy Fuels 4, 199–212 (2020).
Larrazábal, G. O. et al. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces 11, 41281–41288 (2019).
Acknowledgements
This material is based upon work supported by the US Department of Energy under award number DE-FE0031910. We thank the National Science Foundation for financially supporting H.S. (award no. CBET-1803200). We also acknowledge helpful discussions on developing the methodology of the analysis by M. Jouny, and constructive suggestions by S. Overa and B. H. Ko.
Author information
Authors and Affiliations
Contributions
H.S. and K.U.H. contributed equally to this work. H.S., K.U.H. and F.J. performed data analysis and wrote the manuscript. F.J. supervised the whole project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Sustainability thanks Ung Lee and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–8, Figs. 1–13 and Tables 1–14.
Supplementary Data 1
Spreadsheet used for computing production costs.
Supplementary Data 2
MATLAB code used for computing electrolyser voltammetric performance.
Rights and permissions
About this article
Cite this article
Shin, H., Hansen, K.U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat Sustain 4, 911–919 (2021). https://doi.org/10.1038/s41893-021-00739-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41893-021-00739-x
This article is cited by
-
Integrated energy storage and CO2 conversion using an aqueous battery with tamed asymmetric reactions
Nature Communications (2024)
-
Engineering redox-active electrochemically mediated carbon dioxide capture systems
Nature Chemical Engineering (2024)
-
Integrating hydrogen utilization in CO2 electrolysis with reduced energy loss
Nature Communications (2024)
-
Acidic media enables oxygen-tolerant electrosynthesis of multicarbon products from simulated flue gas
Nature Communications (2024)
-
Unraveling the rate-determining step of C2+ products during electrochemical CO reduction
Nature Communications (2024)