Abstract
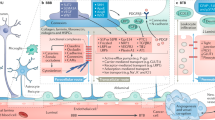
The protective blood–brain barrier has a major role in ensuring normal brain function by severely limiting and tightly controlling the ingress of substances into the brain from the circulation. In primary brain tumours, such as glioblastomas, as well as in brain metastases from cancers in other organs, including lung and breast cancers and melanoma, the blood–brain barrier is modified and is referred to as the blood–tumour barrier (BTB). Alterations in the BTB affect its permeability, and this structure participates in reciprocal regulatory pathways with tumour cells. Importantly, the BTB typically retains a heterogeneous capacity to restrict the penetration of many therapeutic agents into intracranial tumours, and overcoming this challenge is a key to improving the effectiveness of treatment and patient quality of life. Herein, current knowledge of BTB structure and function is reviewed from a cell and cancer biology standpoint, with a focus on findings derived from in vivo models and human tumour specimens. Additionally, how this knowledge can be translated into clinical advances for patients with cancer is discussed.
Key points
-
The blood–brain barrier surrounding capillaries in the brain parenchyma is modified to a blood–tumour barrier (BTB) upon the development of primary or metastatic brain tumours.
-
The BTB is heterogeneously permeable to many drugs, contributing to poor therapeutic efficacy.
-
Research has uncovered consistent changes in BTBs. BTB permeability has been changed by modifying select alterations, indicating that the BTB presents viable molecular targets for improving drug efficacy.
-
New brain-penetrant molecularly targeted inhibitors of oncoproteins have demonstrated efficacy in subgroups of patients with brain metastasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 21 (Suppl. 5), v1–v100 (2019).
Larjavaara, S. et al. Incidence of gliomas by anatomic location. Neuro-oncology 9, 319–325 (2007).
Weller, M. et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 18, e315–e329 (2017).
Walid, M. S. Prognostic factors for long-term survival after glioblastoma. Perm. J. 12, 45–48 (2008).
Valiente, M. et al. The evolving landscape of brain metastasis. Trends Cancer 4, 176–196 (2018).
Boire, A., Brastianos, P. K., Garzia, L. & Valiente, M. Brain metastasis. Nat. Rev. Cancer 20, 4–11 (2020).
Peters, S. et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377, 829–838 (2017).
Wu, Y. L. et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 383, 1711–1723 (2020).
Park, S. et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann. Oncol. 31, 1397–1404 (2020).
Brufsky, A. M. et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin. Cancer Res. 17, 4834–4843 (2011).
Hurvitz, S. A. et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin. Cancer Res. 25, 2433–2441 (2019).
Lin, N. U. et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 38, 2610–2619 (2020).
Amaral, T. et al. Combined immunotherapy with nivolumab and ipilimumab with and without local therapy in patients with melanoma brain metastasis: a DeCOG* study in 380 patients. J. Immunother. Cancer 8, e000333 (2020).
Tawbi, H. A. et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N. Engl. J. Med. 379, 722–730 (2018).
van Tellingen, O. et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 19, 1–12 (2015).
Erlich, P. D. Das Sauerstoff-Bedürfniss des Organismus (Hirschwald, 1885).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
Pardridge, W. M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 13, 963–975 (2016).
Daneman, R. & Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412 (2015).
Arvanitis, C., Ferraro, G. & Jain, R. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41 (2020).
Vanlandewijck, M. et al. Author Correction: A molecular atlas of cell types and zonation in the brain vasculature. Nature 560, E3 (2018).
Keaney, J. & Campbell, M. The dynamic blood-brain barrier. FEBS J. 282, 4067–4079 (2015).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–U238 (2010).
Villaseñor, R., Lampe, J., Schwaninger, M. & Collin, L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell Mol. Life Sci. 76, 1081–1092 (2019).
Ben-Zvi, A. et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511 (2014).
Miller, D. S. in ABC Transporters and Cancer Vol. 125 (eds Ishikawa, T. & Schuetz, J. D.) 43–70 (Academic, 2015).
Liebner, S. et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 183, 409–417 (2008).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Zhou, W. C. et al. Targeting glioma stem cell-derived pericytes disrupts the blood-tumor barrier and improves chemotherapeutic efficacy. Cell Stem Cell 21, 591–603.e4 (2017).
Yao, Y., Chen, Z. L., Norris, E. H. & Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 5, 3413 (2014).
Zhou, J. P. et al. Altered blood-brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport 19, 1–5 (2008).
Abbott, N. J., Ronnback, L. & Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
Zhao, Z., Nelson, A. R., Betsholtz, C. & Zlokovic, B. V. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078 (2015).
Saito, S. & Ihara, M. Interaction between cerebrovascular disease and Alzheimer pathology. Curr. Opin. Psychiatry 29, 168–173 (2016).
Carare, R. O. et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 34, 131–144 (2008).
Rennels, M. L., Gregory, T. F., Blaumanis, O. R., Fujimoto, K. & Grady, P. A. Evidence for a paravascular fluid circulation in the mammalian central nervous-system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 326, 47–63 (1985).
Carbonell, W. S., Ansorge, O., Sibson, N. & Muschel, R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4, e5857 (2009).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Fitzgerald, D. et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metast. 25, 799–810 (2008).
Hambardzumyan, D. & Bergers, G. Glioblastoma: defining tumor niches. Trends Cancer 1, 252–265 (2015).
Silver, J. & Miller, J. H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 5, 146–156 (2004).
Gril, B. et al. Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat. Commun. 9, 2705 (2018).
Kierdorf, K., Masuda, T., Jordao, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Ratnam, N. M., Gilbert, M. R. & Giles, A. J. Immunotherapy in CNS cancers: the role of immune cell trafficking. Neuro-oncology 21, 37–46 (2019).
Duchnowska, R. et al. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 18, 43 (2016).
Russo, M. V. & McGavern, D. B. Immune Surveillance of the CNS following infection and Injury. Trends Immunol. 36, 637–650 (2015).
Harter, P. N. et al. Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 6, 40836–40849 (2015).
Gargini, R., Segura-Collar, B. & Sanchez-Gomez, P. Cellular plasticity and tumor microenvironment in gliomas: the struggle to hit a moving target. Cancers 12, 24 (2020).
Hoque, M. M. et al. The cerebral microvasculature: basic and clinical perspectives on stroke and glioma. Microcirculation 28, e12671 (2021).
Baisiwala, S. et al. Chemotherapeutic stress induces transdifferentiation of glioblastoma cells to endothelial cells and promotes vascular mimicry. Stem Cell Int. 2019, 14 (2019).
Teglasi, V. et al. Origin and distribution of connective tissue and pericytes impacting vascularization in brain metastases with different growth patterns. J. Neuropathol. Exp. Neurol. 78, 326–339 (2019).
Lockman, P. R. et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 16, 5664–5678 (2010).
Bugyik, E. et al. Lack of angiogenesis in experimental brain metastases. J. Neuropathol. Exp. Neurol. 70, 979–991 (2011).
Lyle, L. et al. Alterations in pericyte subpopulations are associated with elevated blood-tumor barrier permeability in experimental brain metastasis of breast cancer. Clin. Cancer Res. 22, 5287–5299 (2016).
Kealy, J. & Campbell, M. in Resistance to Targeted Therapies against Adult Brain Cancers Vol. 11 (ed Tivnan, A.) 69–87 (Springer, 2016).
Avraham, H. K. et al. Angiopoietin-2 mediates blood-brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J. Pathol. 232, 369–381 (2014).
Rodriguez, P. L., Jiang, S. X., Fu, Y. G., Avraham, S. & Avraham, H. K. The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int. J. Cancer 134, 1034–1044 (2014).
Wolburg, H. et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 105, 586–592 (2003).
Bao, X. et al. Protein expression and functional relevance of efflux and uptake drug transporters at the blood-brain barrier of human brain and glioblastoma. Clin. Pharmacol. Ther. 107, 1116–1127 (2020).
Phoenix, T. N. et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29, 508–522 (2016).
Connell, J. J. et al. Selective permeabilization of the bloodbrain barrier at sites of metastasis. J. Natl Cancer Inst. 105, 1634–1643 (2013).
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596 (2013).
Bao, X. et al. Quantitative protein expression of blood-brain barrier transporters in the vasculature of brain metastases of patients with lung and breast cancer. Clin. Transl. Sci. https://doi.org/10.1111/cts.12978 (2021).
Bronger, H. et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 65, 11419–11428 (2005).
Tiwary, S. et al. Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci. Rep. 8, 8267 (2018).
Pernet-Gallay, K. et al. Vascular permeability in the RG2 glioma model can be mediated by macropinocytosis and be independent of the opening of the tight junction. J. Cereb. Blood Flow Metab. 37, 1264–1275 (2017).
Gril, B. et al. A HER2 antibody drug conjugate controls growth of breast cancer brain metastasis in hematogenous xenograft models, with heterogeneous blood-tumor barrier penetration unlinked to a passive marker. Neuro-oncology 22, 1625–1636 (2020).
Lee, S. T. et al. Loss of pericytes in radiation necrosis after glioblastoma treatments. Mol. Neurobiol. 55, 4918–4926 (2018).
Valdor, R. et al. Glioblastoma progression is assisted by induction of immunosuppressive function of pericytes through interaction with tumor cells. Oncotarget 8, 68614–68626 (2017).
Miroshnikova, Y. A. et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 18, 1336–1345 (2016).
Morad, G. et al. Cdc42-dependent transfer of mir301 from breast cancer-derived extracellular vesicles regulates the matrix modulating ability of astrocytes at the blood-brain barrier. Int. J. Mol. Sci. 21, 17 (2020).
Nduom, E. K., Yang, C. Z., Merrill, M. J., Zhuang, Z. P. & Lonser, R. R. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms Laboratory investigation. J. Neurosurg. 119, 427–433 (2013).
Brandao, M., Simon, T., Critchley, G. & Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 67, 779–790 (2019).
Wasilewski, D., Priego, N., Fustero-Torre, C. & Valiente, M. Reactive astrocytes in brain metastasis. Front. Oncol. 7, 298 (2017).
Doron, H., Pukrop, T. & Erez, N. A blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res. 79, 423–436 (2019).
Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10, 459–466 (2009).
Brastianos, P. K. et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Disc. 5, 1164–1177 (2015).
Samala, R. et al. Vinorelbine delivery and efficacy in the MDA-MB-231BR preclinical model of brain metastases of breast cancer. Mol. Pharmacol. 33, 2904–2919 (2016).
Askoxylakis, V. et al. Preclinical efficacy of ado-trastuzumab emtansine in the brain microenvironment. J. Natl Cancer Inst. 108, 10 (2016).
Terrell-Hall, T. B., Nounou, M. I., El-Amrawy, F., Griffith, J. I. G. & Lockman, P. R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 8, 83734–83744 (2017).
Taskar, K. S. et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm. Res. 29, 770–781 (2012).
Nounou, M. I. et al. Anti-cancer antibody trastuzumab-melanotransferrin conjugate (BT2111) for the treatment of metastatic HER2+breast cancer tumors in the brain: an in-vivo study. Pharm. Res. 33, 2930–2942 (2016).
Zhou, Q. Y. et al. Predicting human tumor drug concentrations from a preclinical pharmacokinetic model of temozolomide brain disposition. Clin. Cancer Res. 13, 4271–4279 (2007).
Hasegawa, H., Ushio, Y., Hayakawa, T., Yamada, K. & Mogami, H. Changes of the blood-brain-barrier in experimental metastatic brain-tumors. J. Neurosurg. 59, 304–310 (1983).
Osswald, M. et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin. Cancer Res. 22, 6078–6087 (2016).
Thomas, F. C. et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 26, 2486–2494 (2009).
Li, Y., Zheng, X. M., Gong, M. & Zhang, J. N. Delivery of a peptide-drug conjugate targeting the blood brain barrier improved the efficacy of paclitaxel against glioma. Oncotarget 7, 79387–79393 (2016).
Regina, A. et al. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol. Cancer Ther. 14, 129–140 (2015).
Mittapalli, R. K. et al. Paclitaxel-hyaluronic nanoconjugates prolong overall survival in a preclinical brain metastases of breast cancer model. Mol. Cancer Ther. 12, 2389–2399 (2013).
Mohammad, A. S. et al. Liposomal irinotecan accumulates in metastatic lesions, crosses the blood–tumor barrier (BTB), and prolongs survival in an experimental model of brain metastases of triple negative breast cancer. Pharm. Res. 35, 31 (2018).
Henry, M. N., Chen, Y. H., McFadden, C. D., Simedrea, F. C. & Foster, P. J. In-vivo longitudinal MRI study: an assessment of melanoma brain metastases in a clinically relevant mouse model. Melanoma Res. 25, 127–137 (2015).
Murrell, D. H. et al. Understanding heterogeneity and permeability of brain metastases in murine models of HER2-positive breast cancer through magnetic resonance imaging: implications for detection and therapy. Transl. Oncol. 8, 176–184 (2015).
Murrell, D. H. et al. Evaluating changes to blood-brain barrier integrity in brain metastasis over time and after radiation treatment. Transl. Oncol. 9, 219–227 (2016).
Crowe, W. et al. MRI evaluation of the effects of whole brain radiotherapy on breast cancer brain metastasis. Int. J. Radiat. Biol. 95, 338–346 (2018).
Thorsen, F. et al. Multimodal imaging enables early detection and characterization of changes in tumor permeability of brain metastases. J. Control. Rel. 172, 812–822 (2013).
Liu, L. B., Xue, Y. X. & Liu, Y. H. Bradykinin increases the permeability of the blood-tumor barrier by the caveolae-mediated transcellular pathway. J. Neurooncol. 99, 187–194 (2010).
Su, B. X. et al. Effect of retro-inverso isomer of bradykinin on size-dependent penetration of blood-brain tumor barrier. Small 14, 1702331 (2018).
Morikawa, A. et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro-oncology 17, 289–295 (2015).
Nakagawa, H. et al. CIS-diamminedichloroplatinum (CDDP) therapy for brain metastasis of lung cancer. I. Distribution within the central-nervous-system after intravenous and intracarotid infusion. J. Neurooncol. 16, 61–67 (1993).
Portnow, J. et al. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin. Cancer Res. 15, 7092–7098 (2009).
Stewart, D. J. et al. Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J. Neurooncol. 2, 133–139 (1984).
Hofer, S. & Frei, K. Gefitinib concentrations in human glioblastoma tissue. J. Neurooncol. 82, 175–176 (2007).
Green, R. M. et al. Human central nervous-system and plasma pharmacology of mitoxantrone. J. Neurooncol. 6, 75–83 (1988).
Whittle, I. R., Malcolm, G., Jodrell, D. I. & Reid, M. Platinum distribution in malignant glioma following intraoperative intravenous infusion of carboplatin. Br. J. Neurosurg. 13, 132–137 (1999).
Fine, R. L. et al. Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin. Cancer Res. 12, 5770–5776 (2006).
Sasada, S. et al. Cu-64-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Ann. Oncol. 28, 2028–2029 (2017).
Dijkers, E. C. et al. Biodistribution of Zr-89-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 87, 586–592 (2010).
Pokorny, J. L. et al. The efficacy of the wee1 inhibitor MK-1775 combined with temozolomide is limited by heterogeneous distribution across the blood-brain barrier in glioblastoma. Clin. Cancer Res. 21, 1916–1924 (2015).
Li, J. et al. Quantitative and mechanistic understanding of AZD1775 penetration across human blood-brain barrier in glioblastoma patients using an IVIVE-PBPK modeling approach. Clin. Cancer Res. 23, 7454–7466 (2017).
Gilbert, M. R. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 699–708 (2014).
Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108 (2014).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Murthy, R. et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 19, 880–888 (2018).
Liu, Y. et al. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro-oncology 15, 891–903 (2013).
Sorensen, J. B., Hansen, H. H., Hansen, M. & Dombernowsky, P. Brain metastases in adenocarcinoma of the lung — frequency, risk groups, and prognosis. J. Clin. Oncol. 6, 1474–1480 (1988).
Chang, W. Y. et al. The impact of EGFR mutations on the incidence and survival of stages I to III NSCLC patients with subsequent brain metastasis. PLoS ONE 13, e0192161 (2018).
Varrone, A. et al. A PET study in healthy subjects of brain exposure of C-11-labelled osimertinib - A drug intended for treatment of brain metastases in non-small cell lung cancer. J. Cereb. Blood Flow Metab. 40, 799–807 (2020).
Ballard, P. et al. Preclinical Comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res. 22, 5130–5140 (2016).
van Hoppe, S. et al. Brain accumulation of osimertinib and its active metabolite AZ5104 is restricted by ABCB1 (P-glycoprotein) and ABCG2 (breast cancer resistance protein). Pharmacol. Res. 146, 9 (2019).
Higuchi, T. et al. Osimertinib regresses an EGFR-mutant cisplatinum-resistant lung adenocarcinoma growing in the brain in nude mice. Transl. Oncol. 12, 640–645 (2019).
Soria, J. C. et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N. Engl. J. Med. 378, 113–125 (2018).
Costa, D. B. et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J. Clin. Oncol. 33, 1881–U1841 (2015).
Gadgeel, S. M. et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 15, 1119–1128 (2014).
Kodama, T. et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother. Pharmacol. 74, 1023–1028 (2014).
Kroll, R. et al. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: a comaprison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery 43, 879–886 (1998).
Doolittle, N. et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 88, 637–647 (2000).
Karmur, B. S. et al. Blood-brain barrier disruption in neuro-oncology: strategies, failures, and challenges to overcome. Front. Oncol. 10, 563840 (2020).
Lin, Y. L., Wu, M. T. & Yang, F. Y. Pharmacokinetics of doxorubicin in glioblastoma multiforme following ultrasound-Induced blood-brain barrier disruption as determined by microdialysis. J. Pharm. Biomed. Anal. 149, 482–487 (2018).
Arvanitis, C. D. et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc. Natl Acad. Sci. USA 115, E8717–E8726 (2018).
Carpentier, A. et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl Med. 8, 343re2 (2016).
Carman, A. J., Mills, J. H., Krenz, A., Kim, D. G. & Bynoe, M. S. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J. Neurosci. 31, 13272–13280 (2011).
Jackson, S. et al. The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 15, 2 (2018).
Hashizume, K. & Black, K. L. Increased endothelial vesicular transport correlates with increased blood-tumor barrier permeability induced by bradykinin and leukotriene C4. J. Neuropathol. Exp. Neurol. 61, 725–735 (2002).
Prados, M. D. et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro-Oncology 5, 96–103 (2003).
Cote, J. et al. Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS ONE 7, 17 (2012).
Kotb, S. et al. Gadolinium-based nanoparticles and radiation therapy for multiple brain melanoma metastases: proof of concept before phase I trial. Theranostics 6, 418–427 (2016).
Verry, C. et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 6, eaay5279 (2020).
Ding, W. C. & Guo, L. Immobilized transferrin Fe3O4@SiO2 nanoparticle with high doxorubicin loading for dual-targeted tumor drug delivery. Int. J. Nanomed. 8, 4631–4638 (2013).
Liu, D. Z. et al. The enhancement of siPLK1 penetration across BBB and its anti glioblastoma activity in vivo by magnet and transferrin co-modified nanoparticle. Nanomedicine 14, 991–1003 (2018).
Soe, Z. C. et al. Transferrin-conjugated polymeric nanoparticle for receptor-mediated delivery of doxorubicin in doxorubicin-resistant breast cancer cells. Pharmaceutics 11, 63 (2019).
Zheng, B. et al. Delivery of antisense oligonucleotide LOR-2501 using transferrin-conjugated polyethylenimine-based lipid nanoparticle. Anticancer Res. 39, 1785–1793 (2019).
Johnsen, K. B., Burkhart, A., Thomsen, L. B., Andresen, T. L. & Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 181, 101665 (2019).
Yu, Y. J. et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl Med. 3, 84ra44 (2011).
Ruan, S. B. et al. Acid-responsive transferrin dissociation and GLUT mediated exocytosis for increased blood-brain barrier transcytosis and programmed glioma targeting delivery. Adv. Funct. Mater. 28, 1802227 (2018).
Regina, A. et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector angiopep-2. Br. J. Pharmacol. 155, 185–197 (2008).
Xin, H. L. et al. Anti-glioblastoma efficacy and safety of paclitaxel-loading angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 33, 8167–8176 (2012).
Kumthekar, P. et al. ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin. Cancer Res. 26, 2789–2799 (2020).
Hong, S. S., Oh, K. T., Choi, H. G. & Lim, S. J. Liposomal formulations for nose-to-brain delivery: recent advances and future perspectives. Pharmaceutics 11, 540 (2019).
Vieira, D. B. & Gamarra, L. F. Getting into the brain: liposome-based strategies for effective drug delivery across the blood-brain barrier. Int. J. Nanomed. 11, 5381–5414 (2016).
Mok, T. S. et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 376, 629–640 (2017).
Ramalingam, S. S. et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N. Engl. J. Med. 382, 41–50 (2020).
Zimmer, A. S. et al. Temozolomide in secondary prevention of HER2-positive breast cancer brain metastases. Future Oncol. 16, 899–909 (2020).
Palmieri, D. et al. Profound prevention of experimental brain metastases of breast cancer by temozolomide in an MGMT-dependent manner. Clin. Cancer Res. 20, 2727–2739 (2014).
Mittapalli, R. K. et al. Quantitative fluorescence microscopy measures vascular pore size in primary and metastatic brain tumors. Cancer Res. 77, 238–246 (2017).
Shah, N. et al. Drug resistance occurred in a newly characterized preclinical model of lung cancer brain metastasis. BMC Cancer 20, 292 (2020).
Chaves, C. et al. Characterization of the blood-brain barrier integrity and the brain transport of SN-38 in an orthotopic xenograft rat model of diffuse intrinsic pontine glioma. Pharmaceutics 12, 399 (2020).
Samala, R. et al. Vinorelbine delivery and efficacy in the MDA-MB-231BR preclinical model of brain metastases of breast cancer. Pharm. Res. 33, 2904–2919 (2016).
Liu, H. L. et al. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS ONE 9, e114311 (2014).
Becker, C. M. et al. Decreased affinity for efflux transporters increases brain penetrance and molecular targeting of a PI3K/mTOR inhibitor in a mouse model of glioblastoma. Neuro-Oncology 17, 1210–1219 (2015).
Phillips, G. D. L. et al. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res. Treat. 164, 581–591 (2017).
Valiente, M. et al. Brain metastasis cell lines panel: a public resource of organotropic cell lines. Cancer Res. 80, 4314–4323 (2020).
Cruz, W. A. et al. Development of a preclinical model of spontaneous human melanoma CNS metastasis. Clin. Exp. Metastasis 26, 930 (2009).
Vaubel, R. A. et al. Genomic and phenotypic characterization of a broad panel of patient-derived xenografts reflects the diversity of glioblastoma. Clin. Cancer Res. 26, 1094–1104 (2020).
Helms, H. C. et al. In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 36, 862–890 (2016).
Acknowledgements
The author thanks M. Gilbert, A. Zimmer, W. D. Figg, B. Gril, I. Khan and S. Lipkowitz for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks Twan Lammers, Seyed Alireza Mansouri, Timothy Phoenix and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Steeg, P.S. The blood–tumour barrier in cancer biology and therapy. Nat Rev Clin Oncol 18, 696–714 (2021). https://doi.org/10.1038/s41571-021-00529-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00529-6
This article is cited by
-
Proteomics study of primary and recurrent adamantinomatous craniopharyngiomas
Clinical Proteomics (2024)
-
Understanding the immunosuppressive microenvironment of glioma: mechanistic insights and clinical perspectives
Journal of Hematology & Oncology (2024)
-
From pre-clinical to translational brain metastasis research: current challenges and emerging opportunities
Clinical & Experimental Metastasis (2024)
-
Clinical Management of Patients with Non-Small Cell Lung Cancer, Brain Metastases, and Actionable Genomic Alterations: A Systematic Literature Review
Advances in Therapy (2024)
-
Blood–Brain Barrier Disruption for the Treatment of Primary Brain Tumors: Advances in the Past Half-Decade
Current Oncology Reports (2024)