Abstract
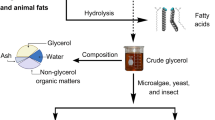
This research aimed to apply the acidification method to remove impurities from biodiesel-derived crude glycerol (BCG) and investigate the potential of purified BCG (PBCG) to be used as a carbon source for microbial oil production by oleaginous yeast Pseudozyma parantarctica CHC28. The optimum purifying conditions of BCG were pretreated with hydrochloric acid at a pH of 1.71. The PBCG concentration was 469.52 g/L under optimized conditions. The batch fermentation was studied using a nitrogen-limited medium containing PBCG (PBCG-medium). The PBCG-medium with 50 g of PBCG per liter provided the maximum biomass and oil concentration of 11.10 g/L and 4.07 g/L, respectively. The oil accumulation was approximately 45%. The long-chain fatty acids C16–C18 were the main compositions, which accounted for over 85%. These results suggested that the conversion of PBCG into microbial oil was an interesting direction to produce microbial oil. It was also a remarkable solution to add value to the by-products from biodiesel production. The mathematical model was demonstrated to describe satisfactorily the yeast growth and microbial oil production profile achieved in media containing pure glycerol and PBCG as carbon sources.


Similar content being viewed by others
Abbreviations
- PG:
-
Pure glycerol
- BCG:
-
Biodiesel-derived crude glycerol
- PBCG:
-
Purified biodiesel-based crude glycerol
- X 1 :
-
Types of acid
- X 2 :
-
PH level
- Y :
-
Glycerol concentration (g/L)
- \({X}_{\text{i}}\), \({X}_{\text{j}}\) :
-
Coded independent variable
- FFAs:
-
Free fatty acids
- dX/dt :
-
The rate of growth (g/L h)
- dS/dt :
-
The rate of substrate consumption (g/L h)
- dP/dt :
-
The rate of oil production (g/L h)
- X :
-
Biomass concentration (g/L)
- X max :
-
Maximum biomass concentration (g/L)
- S :
-
Substrate concentration (g/L)
- ∆ S :
-
Consumed substrate (g/L)
- P :
-
Oil concentration (g/L)
- Y x/s :
-
Biomass yield (g cell/g substrate)
- Y p/s :
-
Product yield (g oil /g substrate)
- Y p/x :
-
Oil content (g oil/g cell)
- k i :
-
Substrate inhibition coefficient (g/L)
- m s :
-
Biomass maintenance coefficient (g/g h)
- R 2 :
-
Coefficient of determination (dimensionless)
- NRMS:
-
Non-normalized root mean square (dimensionless)
- N :
-
Number of measurements
- \({q}_{\text{cal}}\) :
-
Calculated value of a variable from the model (g/L)
- \({q}_{\text{exp}}\) :
-
Experimental value of a variable (g/L)
- \({\stackrel{\mathrm{-}}{{q}}}_{\text{exp}}\) :
-
Average of all the experimental values of a variable (g/L)
- CN:
-
Cetane number
- HHV:
-
High heating value
- KV:
-
Kinematic viscosity
- D:
-
Density
- SV:
-
Saponification value
- IV:
-
Iodine value
- β 0 :
-
Intercept coefficients of quadratic equation
- β i :
-
Linear coefficients of quadratic equation
- β ij :
-
Interaction coefficients of quadratic equation
- β ii :
-
Squared coefficient of the quadratic equation
- µ :
-
The specific growth rate (h−1)
- µ max :
-
The maximum specific growth rate (h−1)
- α :
-
Growth-associated coefficient for the Luedeking-Piret (g/g)
- β :
-
Non-growth associated coefficient for the Luedeking-Piret (g/g h)
References
Cui Y, Blackburn JW, Liang Y (2012) Fermentation optimization for the production of lipid by Cryptococcus curvatus: Use of response surface methodology. Biomass Bioenergy 47:410–417. https://doi.org/10.1016/j.biombioe.2012.09.017
Dobrowolski A, Mituła P, Rymowicz W, Mirończuk AM (2016) Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour Technol 207:237–243. https://doi.org/10.1016/j.biortech.2016.02.039
Nasir NF, Mirus MF, Ismail M (2017) Purification of crude glycerol from transesterification reaction of palm oil using direct method and multistep method. IOP Conf Ser Mater Sci Eng 243:012015. https://doi.org/10.1088/1757-899X/243/1/012015
Pitt FD, Domingos AM, Barros AAC (2019) Purification of residual glycerol recovered from biodiesel production. South Afr J Chem Eng 29:42–51. https://doi.org/10.1016/j.sajce.2019.06.001
Vicente G, Martínez M, Aracil J (2007) Optimisation of integrated biodiesel production. Part II: a study of the material balance. Bioresour Technol 98:1754–1761. https://doi.org/10.1016/j.biortech.2006.07.023
Adnan NAA, Suhaimi SN, Abd-Aziz S, Hassan MA, Phang LY (2014) Optimization of bioethanol production from glycerol by Escherichia coli SS1. Renew Energy 66:625–633. https://doi.org/10.1016/j.renene.2013.12.032
Johnson DT, Taconi KA (2007) The glycerin glut: options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ Prog 26:338–348. https://doi.org/10.1002/ep.10225
Wan Isahak WNR, Che Ramli ZA, Ismail M, Mohd Jahim J, Yarmo MA (2015) Recovery and purification of crude glycerol from vegetable oil transesterification. Sep Purif Rev 44:250–267. https://doi.org/10.1080/15422119.2013.851696
Nanda MR, Yuan Z, Qin W, Poirier MA, Chunbao X (2014) Purification of crude glycerol using acidification: effects of acid types and product characterization. Austin J Chem Eng 1:1–7
Yong KC, Ooi TL, Dzulkefly K, Wan Yunus WMZ, Hazimah AH (2001) Characterization of glycerol residue from a palm kernel oil methyl ester plant. J Oil Palm Res 13:1–6
Kongjao S, Damronglerd S, Hunsom M (2010) Purification of crude glycerol derived from waste used-oil methyl ester plant. Korean J Chem Eng 27:944–949. https://doi.org/10.1007/s11814-010-0148-0
Manowattana A, Techapun C, Watanabe M, Chaiyaso T (2018) Bioconversion of biodiesel-derived crude glycerol into lipids and carotenoids by an oleaginous red yeast Sporidiobolus pararoseus KM281507 in an airlift bioreactor. J Biosci Bioeng 125:59–66. https://doi.org/10.1016/j.jbiosc.2017.07.014
Kitcha S, Cheirsilp B (2012) Enhancing lipid production from crude glycerol by newly Isolated oleaginous yeasts: strain selection, process optimization, and fed-batch strategy. BioEnergy Res 6:300–310. https://doi.org/10.1007/s12155-012-9257-4
Thanapimmetha A, Suwaleerat T, Saisriyoot M, Limtong S (2017) Production of carotenoids and lipids by Rhodococcus opacus PD630 in batch and fed-batch culture. Bioprocess Biosyst Eng 40:133–143. https://doi.org/10.1007/s00449-016-1681-y
Rane DV, Pawar PP, Odaneth AA, Lali AM (2021) Microbial oil production by the oleaginous red yeast, Rhodotorula glutinis NCIM 3168, using corncob hydrolysate. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01298-z
Poontawee R, Yongmanitchai W, Limtong S (2018) Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis. Process Biochem 66:150–161. https://doi.org/10.1016/j.procbio.2017.11.020
Cui Y, Blackburn JW, Liang Y (2012) Fermentation optimization for the production of lipid by Cryptococcus curvatus: use of response surface methodology. Biomass Bioenergy 47:410–417. https://doi.org/10.1016/j.biombioe.2012.09.017
Polburee P, Yongmanitchai W, Honda K, Ohashi T, Ohashi T, Fujiyama K, Limtong S (2016) Lipid production from biodiesel-derived crude glycerol by Rhodosporidium fluviale DMKU-RK253 using temperature shift with high cell density. Biochem Eng J 112:208–218. https://doi.org/10.1016/j.bej.2016.04.024
Areesirisuk A, Chiu CH, Yen SB, Guo JH (2015) A novel oleaginous yeast strain with high lipid productivity and its application to alternative biodiesel production. Appl Biochem Microbiol 51:411–418
Manosak R, Limpattayanate S, Hunsom M (2011) Sequential-refining of crude glycerol derived from waste used-oil methyl ester plant via a combined process of chemical and adsorption. Fuel Process Technol 92:92–99. https://doi.org/10.1016/j.fuproc.2010.09.002
Cescut J, Severac E, Molina-Jouve C, Uribelarrea J-L (2011) Optimizing pressurized liquid extraction of microbial lipids using the response surface method. J Chromatogr A 1218:373–379. https://doi.org/10.1016/j.chroma.2010.12.003
Ghorbel-Bellaaj O, Hmidet N, Jellouli K, Younes I, Maâlej H, Hachicha R, Nasri M (2011) Shrimp waste fermentation with Pseudomonas aeruginosa A2: optimization of chitin extraction conditions through Plackett-Burman and response surface methodology approaches. Int J Biol Macromol 48:596–602. https://doi.org/10.1016/j.ijbiomac.2011.01.024
Saran S, Mathur A, Dalal J, Saxena RK (2017) Process optimization for cultivation and oil accumulation in an oleaginous yeast Rhodosporidium toruloides A29. Fuel 188:324–331. https://doi.org/10.1016/j.fuel.2016.09.051
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Bondioli P, Bella LD (2005) An alternative spectrophotometric method for the determination of free glycerol in biodiesel. Eur J Lipid Sci Technol 107:153–157. https://doi.org/10.1002/ejlt.200401054
Feng J, Zhang J-S, Jia W, Yang Y, Liu F, Lin CC (2014) An unstructured kinetic model for the improvement of triterpenes production by Ganoderma lucidum G0119 based on nitrogen source effect. Biotechnol Bioprocess Eng 19:727–732. https://doi.org/10.1007/s12257-014-0049-x
Marudkla J, Lee W-C, Wannawilai S, Chisti Y, Sirisansaneeyakul S (2018) Model of acetic acid-affected growth and poly(3-hydroxybutyrate) production by Cupriavidus necator DSM 545. J Biotechnol 268:12–20. https://doi.org/10.1016/j.jbiotec.2018.01.004
Yang J, Rasa E, Tantayotai P, Scow KM, Yuan H, Hristova KR (2011) Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Bioresour Technol 102:3077–3082. https://doi.org/10.1016/j.biortech.2010.10.049
Kumar KV (2007) Pseudo-second order models for the adsorption of safranin onto activated carbon: comparison of linear and non-linear regression methods. J Hazard Mater 142:564–567. https://doi.org/10.1016/j.jhazmat.2006.08.018
Yang X, Al-Duri B (2005) Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon. J Colloid Interface Sci 287:25–34. https://doi.org/10.1016/j.jcis.2005.01.093
Sadhukhan S, Sarkar U (2016) Production of purified glycerol using sequential desalination and extraction of crude glycerol obtained during trans-esterification of Crotalaria juncea oil. Energy Convers Manag 118:450–458. https://doi.org/10.1016/j.enconman.2016.03.088
Hajek M, Skopal F (2010) Treatment of glycerol phase formed by biodiesel production. Bioresour Technol 101:3242–3245. https://doi.org/10.1016/j.biortech.2009.12.094
Chatzifragkou A, Makri A, Belka A, Bellou S, Mavrou M, Mastoridou M, Mystrioti P, Onjaro G, Aggelis G, Papanikolaou S (2011) Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 36:1097–1108. https://doi.org/10.1016/j.energy.2010.11.040
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011) Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem 46:210–218. https://doi.org/10.1016/j.procbio.2010.08.009
Karamerou EE, Theodoropoulos C, Webb C (2016) A biorefinery approach to microbial oil production from glycerol by Rhodotorula glutinis. Biomass Bioenergy 89:113–122. https://doi.org/10.1016/j.biombioe.2016.01.007
Yen H-W, Yang Y-C, Yu Y-H (2012) Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J Biosci Bioeng 114:453–456. https://doi.org/10.1016/j.jbiosc.2012.04.022
Rywińska A, Rymowicz W, Marcinkiewicz M (2010) Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron J Biotechnol 13:4. https://doi.org/10.2225/vol13-issue4-fulltext-1
Raimondi S, Rossi M, Leonardi A, Bianchi MM, Rinaldi T, Amaretti A (2014) Getting lipids from glycerol: new perspectives on biotechnological exploitation of Candida freyschussii. Microb Cell Factories 13:83. https://doi.org/10.1186/1475-2859-13-83
Meesters PAEP, Huijberts GNM, Eggink G (1996) High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl Microbiol Biotechnol 45:575–579. https://doi.org/10.1007/s002530050731
Papanikolaou S, Aggelis G (2002) Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresour Technol 82:43–49. https://doi.org/10.1016/S0960-8524(01)00149-3
Liang Y, Cui Y, Trushenski J, Blackburn JW (2010) Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour Technol 101:7581–7586. https://doi.org/10.1016/j.biortech.2010.04.061
Uçkun Kiran E, Trzcinski A, Webb C (2013) Microbial oil produced from biodiesel by-products could enhance overall production. Bioresour Technol 129:650–654. https://doi.org/10.1016/j.biortech.2012.11.152
Qureshi N, Saha BC, Cotta MA (2007) Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst Eng 30:419–427. https://doi.org/10.1007/s00449-007-0137-9
Birgen C, Berglihn OT, Preisig HA, Wentzel A (2019) Kinetic study of butanol production from mixtures of glucose and xylose and investigation of different pre-growth strategies. Biochem Eng J 147:110–117. https://doi.org/10.1016/j.bej.2019.04.002
Ryu B-G, Kim J, Kim K, Choi YE, Han JI, Yang JW (2013) High-cell-density cultivation of oleaginous yeast Cryptococcus curvatus for biodiesel production using organic waste from the brewery industry. Bioresour Technol 135:357–364. https://doi.org/10.1016/j.biortech.2012.09.054
Ramírez-Verduzco LF, Rodríguez-Rodríguez JE, Jaramillo-Jacob del Rayo A (2012) Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 91:102–111. https://doi.org/10.1016/j.fuel.2011.06.070
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sustain Energy Rev 16:143–169. https://doi.org/10.1016/j.rser.2011.07.143
Acknowledgements
We acknowledge the Rajamangala University of Technology Thanyaburi (RMUTT) annual government statement of expenditure in 2017 (Grant No. 349185) for financial support.
Funding
The research was supported by the Rajamangala University of Technology Thanyaburi (RMUTT) annual government statement of expenditure in 2017 (Grant No. 349185).
Author information
Authors and Affiliations
Contributions
Atsadawut Areesirisuk and Jantima Teeka contributed to the study conception and design. Chutima Rakkitkanphun and Atsadawut Areesirisuk performed material preparation, data collection and analysis, and writing the first draft of the manuscript. Dolnapa Kaewpa performed data analysis. All authors commented on previous versions of the manuscript. Atsadawut Areesirisuk read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Applicable.
Consent for publication
Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rakkitkanphun, C., Teeka, J., Kaewpa, D. et al. Purification of biodiesel-derived crude glycerol by acidification to be used as a carbon source for microbial oil production by oleaginous yeast Pseudozyma parantarctica CHC28. Biomass Conv. Bioref. 13, 15381–15391 (2023). https://doi.org/10.1007/s13399-021-01600-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01600-z