Abstract
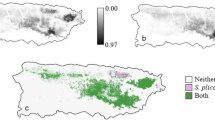
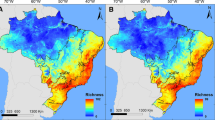
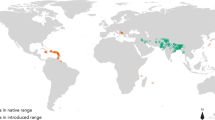
Establishment of new populations is contingent on overcoming abiotic and biotic barriers. While this applies to all species, these hurdles are at the forefront of invasion biology where prediction, prevention, eradication, and control strategies depend on an understanding of these processes. Terrestrial Arundina graminifolia and epiphytic Dendrobium crumenatum are two non-indigenous orchids spreading throughout Puerto Rico. The two species have acquired a native herbivore and seed predator, the orchid-specialist weevil, Stethobaris polita. With recently acquired presence records of the three species, land cover data and downscaled climate variables, we modeled their potential distributions under current conditions and also those projected under the least and most extreme climate scenarios for 2050 and 2070. We show that D. crumenatum flourishes in urban environments which also provide refugia from S. polita, whereas there is currently limited refugia for A. graminifolia from S. polita attack, as this orchid has similar climatic niches as the weevil. Projections into all climate scenarios suggest range retractions for all species, with a decreased extent of both orchid populations subject to S. polita attack. Thus, we illustrate for island invasions how climate change will likely alter the distribution of acquired biotic interactions.




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abelleira Martínez OJ, Rodríguez MA, Rosario I, Soto N, López A, Lugo AE (2010) Structure and species composition of novel forests dominated by an introduced species in northcentral Puerto Rico. New for 39:1–18
Ackerman JD (1995) An orchid flora of Puerto Rico and the Virgin Islands. Mem NY Bot Gard 73:1–203
Ackerman JD (2007) Invasive orchids: weeds we hate to love? Lankesteriana 7:19–21
Ackerman JD (2012) Orchids gone wild: discovering naturalized orchids in Hawaii. Orchids 81:88–93
Ackerman JD (2021) Island invasions by introduced honey bees: what can be expected for Puerto Rico and the Caribbean? Front Ecol Evol 8:556744
Ackerman JD, Collaborators, (2014) Orchid flora of the Greater Antilles. Mem NY Bot Gard 109:1–625
Ackerman JD, Falcón W, Molinari J, Vega C, Espino I, Cuevas AA (2014) Biotic resistance and invasional meltdown: consequences of acquired interspecific interactions for an invasive orchid, Spathoglottis plicata in Puerto Rico. Biol Invasions 16:2435–2447
Ackerman JD (2017) Orchidées invasives: acceleration de la colonization et de la propagation. L’Orchidophile 213:167–173
Allendorf FW, Lundquist LL (2003) Introduction: population biology, evolution, and control of invasive species. Conserv Biol 17:24–30
Almasi KN (2000) A non-native perennial invades a native forest. Biol Invasions 2:219–230
Aryal A, Shrestha UB, Ji W, Ale SB, Shrestha S, Ingty T, Maraseni T, Cockfield G, Raubenheimer D (2016) Predicting the distributions of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecol Evol 6:065–4075
Bayman P, Espinosa ATM, Aponte CMS, Guevara NCH, Ruiz NLV (2016) Age-dependent mycorrhizal specificity in an invasive orchid, Oeceoclades maculata. Am J Bot 103:1880–1889
Bayman P, González EJ, Fumero JJ, Tremblay RL (2002) Are fungi necessary? How fungicides affect growth and survival of the orchid Lepanthes rupestris in the field. J Ecol 90:1002–1008
Beaumont LJ, Gallagher RV, Downey PO, Thuiller W, Leishman MR, Hughes L (2009) Modelling the impact of Hieracium spp. on protected areas in Australia under future climates. Ecography 32:757–764
Bellgard SE, Williams SE (2011) Response of mycorrhizal diversity to current climatic changes. Diversity 3:8–90
Benzing DH (1990) Vascular epiphytes. Cambridge University Press, Cambridge, UK
Bradley BA, Oppenheimer M, Wilcove DS (2009) Climate change and plant invasions: restoration opportunities ahead? Glob Change Biol 15:1511–1521
Bradley BA, Blumenthal DM, Wilcove DS, Ziska LH (2010) Predicting plant invasions in an era of global change. Trends Ecol Evol 25:310–318
Brewster LB, Ackerman JD (2013) Distribution of orchid species in the Luquillo Mountains, Puerto Rico. Caribb J Sci 47:50–56
Brooks CJ, Hewitt J (1909) [1910]) Notes on the fertilisation of a few orchids in Sarawak. J Straits Branch R Asiatic Soc 54:99–106
Brown JL, Bennett JR, French CM (2017) SDMtoolbox 2.0: the next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 5:e4095
Burton ML, Samuelson LJ, Pan S (2005) Riparian woody plant diversity and forest structure along an urban-rural gradient. Urban Ecosyst 8:93–106. https://doi.org/10.1007/s11252-005-1421-6
Chen SC, Liu ZJ, Zhu GH et al (2009) Orchidaceae. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China, Science Press, vol 25. Beijing, China, pp 314–315
Chinea JD (2002) Tropical forest succession on abandoned farms in the Humacao Municipality of eastern Puerto Rico. For Ecol Manag 167:195–207
Crain BJ (2012) On the relationship between bryophyte cover and the distribution of Lepanthes spp. Lankesteriana 12:13–18
D’Antonio CM, Dudley TL (1995) Biological invasions as agents of change on islands versus mainlands. In: Vitousek PM, Loope LL, Adsersen H (eds) Islands: Biological diversity and ecosystem function. Springer, Stanford, California, pp 103–121
Daehler CC (1998) Taxonomic distribution of invasive angiosperm plants: ecological insights and comparison to agricultural weeds. Biol Conserv 84:167–180
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Ann Rev Ecol Syst 34:182–211
de Araújo CB, Marcondes-Machado LO, Costa GC (2014) The importance of biotic interactions in species distribution models: a test of the Eltonian noise hypothesis using parrots. J Biogeogr 41:513–523
Dehnen-Schmutz K, Touza J, Perrings C, Williamson M (2007) A century of the ornamental plant trade and its impact on invasion success. Divers Distrib 13:527–534
Denslow JS, Space JC, Thomas PA (2009) Invasive exotic plants in the tropical Pacific islands: patterns of diversity. Biotropica 41:162–170
Dressler RL (1981) The orchids: natural history and classification. Harvard University Press, Cambridge
Dyderski MK, Paź S, Frelich LE, Jagodziński AM (2018) How much does climate change threaten European forest tree species distributions? Glob Change Biol 24:1150–1163
Elith J, Kearney M, Phillips S (2010) The art of modelling range-shifting species. Meth Ecol Evol 1:330–334
Elton CS (1958) The ecology of invasions by animals and plants. Meuthen, London
Ewel JJ, Whitmore JL (1973) The ecological life zones of Puerto Rico and the US Virgin Islands. Research Paper No. ITF-18. Río Piedras: USDA Forest Service Institute of Tropical Forestry
Fagan WF, Lewis M, Neubert MG, Aumann C, Apple JL, Bishop J (2005) When can herbivores slow or reverse the spread of an invading plant? A test case from Mount St. Helens Amer Nat 166:669–685
Falcón W, Ackerman JD, Tremblay RL (2017) Quantifying how acquired interactions with native and invasive insects influence population growth rates of a non-indigenous plant. Biol Invasions 19:895–911
Falcón W, Tremblay RL (2018) From the cage to the wild: introductions of Psittaciformes to Puerto Rico. PeerJ 6:e5669. https://doi.org/10.7717/peerj.5669
Feldmann P, Barré N (2001) Atlas des orchidées sauvages de la Guadeloupe. Collection Patrimoines Naturels 48:1–228
Fick SE, Hijmans RJ (2017) Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315
Forbes HO (1885) On the various contrivances for ensuring self-fertilization in some tropical orchids. Journal of the Linnean Society, Botany 21:538–550
Fine PV, Mesones I, Coley PD (2004) Herbivores promote habitat specialization by trees in Amazonian forests. Science 305:663–665
Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25(6):325–331
Goberville E, Beaugrand G, Hautekèete NC, Piquot Y, Luczak C (2015) Uncertainties in the projection of species distributions related to general circulation models. Ecol Evol 5:1100–1116
Goh CJ, Strauss MS, Arditti J (1982) Flower induction and physiology in orchids. In: Arditti J (ed) Orchid biology: Reviews and perspectives II. Cornell University Press, NY, pp 213–241
Gould WA, Alarcon C, Fevold B, Jimenez ME, Martinuzzi S, Potts G, Quinones M, Mariano S, Ventosa E (2008) The Puerto Rico gap analysis project volume 1: Land cover, vertebrate species, distributions, and land stewardship. USDA Forest Service International Institute of Tropical Forestry
Hayhoe K (2013) Quantifying key drivers of climate variability and change for puerto rico and the caribbean. Raleigh, North Carolina: Caribbean Landscape Conservation Cooperative
Hillerislambers J, Harsch MA, Ettinger AK, Ford KR, Theobald EJ (2013) How will biotic interactions influence climate change–induced range shifts? Ann New York Acad Sci 1297:112–125
Holway DA (1995) Distribution of the Argentine ant (Linepithema humile) in northern California. Conserv Biol 9:1634–1637
Huda MK, Wilcock CC (2012) Rapid floral senescence following male function and breeding systems of some tropical orchids. Plant Biol 14:278–284
Izuddin M, Yam TW, Webb EL (2019) Germination niches and seed persistence of tropical epiphytic orchids in an urban landscape. J Plant Res 132:383–394
Johansen B (1990) Incompatibility in Dendrobium (Orchidaceae). Bot J Linn Soc 103:165–196
Jolliffe K (2010) Epiphytic Orchids of the Seychelles . Kapisen 10:6–8
Khalyani AH, Gould WA, Harmsen E, Terando A, Quinones M, Collazo JA (2016) Climate change implications for tropical islands: Interpolating and interpreting statistically downscaled GCM projections for management and planning. J Appl Meteorol Clim 55:265–282
Kolanowska M, Konowalik K (2014) Niche conservatism and future changes in the potential area coverage of Arundina graminifolia, an invasive orchid species from Southeast Asia. Biotropica 46:157–165
Kolanowska M, Jakubska-Busse A (2020) Is the lady's-slipper orchid (Cypripedium calceolus) likely to shortly become extinct in Europe?—Insights based on ecological niche modelling. PLoS One 15:e0228420.
Lankau RA, Zhu K, Ordonez A (2015) Mycorrhizal strategies of tree species correlate with trailing range edge responses to current and past climate change. Ecology 96:1451–1458
Leong TM, Wee YC (2013) Observations of pollination in the pigeon orchid, Dendrobium crumenatum Swartz (Orchidaceae) in Singapore. Nature Singapore 6:91–96
Ma M, Tan TK, Wong SM (2003) Identification and molecular phylogeny of Epulorhiza isolates from tropical orchids. Mycol Res 107:1041–1049
Mack RN (2003) Global plant dispersal, naturalization, and invasion: pathways, modes, and circumstances. In: Ruiz GM, Carlton JT (eds) Invasive species: vectors and management strategies. Island Press, Washington, pp 3–30
Mack R, Erneberg M (2002) The United States Naturalized Flora: Largely the Product of Deliberate Introductions. Ann Mo Bot Gard 89:176–189. https://doi.org/10.2307/3298562
Malekia M, Sadeghi M (2020) Predicting impacts of climate change on the potential distribution of two interacting species in the forests of western Iran. Meteorol Appl 27:e1800
Martinuzzi S, Gould WA, Gonzalez OMR (2007) Land development, land use, and urban sprawl in Puerto Rico integrating remote sensing and population census data. Lands Urb Plan 79:288–297
McKinney ML (2002) Urbanization, biodiversity, and conservation. Bioscience 52:883–890
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260. https://doi.org/10.1016/j.biocon.2005.09.005
Menéndez R, González-Megías ADELA, Lewis OT, Shaw MR, Thomas CD (2008) Escape from natural enemies during climate-driven range expansion: a case study. Ecol Entomol 33:413–421
Meng Y-Y, Zhang W-O, Selosse M-A, Gao J-Y (2019) Are fungi from adult orchid roots the best symbionts at germination? A case study. Mycorrhiza 29:541–547
Mortensen DA, Rauschert ES, Nord AN, Jones BP (2009) Forest roads facilitate the spread of invasive plants. Invas Plant Sci Mana 2:191–199
Moser B, Fridley JD, Askew AP, Grime JP (2011) Simulated migration in a long-term climate change experiment: invasions impeded by dispersal limitation, not biotic resistance. J Ecol 99:1229–1236
O’Brien CW, Turnbow RH Jr (2011) An annotated list of Curculionidae (Coleoptera) of Dominica (excluding Scolytinae and Platypodidae). Insecta Mundi 0179:1–31
O’Dowd DJ, Green P, Lake PS (2003) Invasional ‘meltdown’ on an oceanic island. Ecol Lett 6:812–817
Olaya-Arenas P, Meléndez-Ackerman EJ, Pérez ME, Tremblay R (2011) Demographic response by a small epiphytic orchid. Amr J Bot 98:2040–2048
Otero JT, Ackerman JD, Bayman P (2002) Diversity and host specificity of mycorrhizal fungi from tropical orchids. Am J Bot 89:1852–1858
Parendes LA, Jones JA (2000) Role of light availability and dispersal in exotic plant invasion along roads and streams in the HJ Andrews Experimental Forest, Oregon. Conser Biol 14:64–75
Phillips SJ, Dudik M, Schapire RE (2018) Maxent software for modeling species niches and distributions (Version 3.4.1). http://biodiversityinformatics.amnh.org/open_source/maxent/. Accessed 22 July 2019
Puerto Rico GAP Analysis Project (2006) PRGAP Landcover. USDA Forest Service, International Institute of Tropical Forestry
Pulwarty RS, Nurse LA, Trotz UO (2010) Caribbean islands in a changing climate. Environment 52(6):16–27
Rasmussen HN, Rasmussen FN (2014) Seedling mycorrhiza: a discussion of origin and evolution in Orchidaceae. Bot J Linn Soc 175:313–327
Recart W, Ackerman JD, Cuevas AA (2013) There goes the neighborhood: apparent competition between invasive and native orchids mediated by a specialist florivorous weevil. Biol Invas 15:283–293
Rojas-Sandoval J, Acevedo-Rodríguez P (2015) Naturalization and invasion of alien plants in Puerto Rico and the Virgin Islands. Biol Invas 17:149–163
Scheffknecht S, Winkler M, Hülber K, Mata Rosas M, Hietz P (2010) Seedling establishment of epiphytic orchids in forests and coffee plantations in Central Veracruz, Mexico. J Trop Ecol 26:93–102
Schweiger O, Settele J, Kudrna O, Klotz S, Kühn I (2008) Climate change can cause spatial mismatch of trophically interacting species. Ecology 89:3472–3479
Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE et al (2017) No saturation in the accumulation of alien species worldwide. Nat Commun. https://doi.org/10.1038/ncomms14435
Seifriz W (1923) The gregarious flowering of the orchid Dendrobium crumenatum. Amer J Bot 10:32–37
Shea K, Chesson P (2002) Community ecology theory as a framework for biological invasions. Trends Ecol Evol 17:170–176
Sin H, Beard KH, Pitt WC (2008) An invasive frog, Eleutherodactylus coqui, increases new leaf production and leaf litter decomposition rates through nutrient cycling in Hawaii. Biol Invasions 10:335–345
Soifer LG, Ackerman JD (2019) Extremes of forest–urban gradient offer some refuge for alien orchid invasion. Biol Invas 21:2143–2157
Stewart AJ, Bantock TM, Beckmann BC, Botham MS, Hubble D, Roy DB (2015) The role of ecological interactions in determining species ranges and range changes. Biol J Linn Soc 115:647–666
Stohlgren TJ, Barnett DT, Jamevich CS, Flather C, Kartesz J (2008) The myth of plant species saturation. Ecol Lett 11:313–326
Sugiura N (2013) Pollination and floral ecology of Arundina graminifolia (Orchidaceae) at the northern border of the species’ natural distribution. J Plant Res 127:131–139
Svenning JC, Gravel D, Holt RD, Schurr FM, Thuiller W, Münkemüller T et al (2014) The influence of interspecific interactions on species range expansion rates. Ecography 37:1198–1209
Thackeray SJ, Sparks TH, Frederiksen M, Burthe S, Bacon PJ, Bell JR et al (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol 16:3304–3313
Thompson J, Lugo AE, Thomlinson J (2007) Land use history, hurricane disturbance, and fate of introduced species in a subtropical wet forest in Puerto Rico. Plant Ecol 192:289–301
Tremblay RL, Ackerman JD, Zimmerman JK, Calvo RN (2005) Variation in sexual reproduction in orchids and its evolutionary consequences: spasmodic journey to diversification. Biol J Lin Soc 84:1–54
Tremblay RL, McCarthy MA (2014) Bayesian estimates of transition probabilities in seven small lithophytic orchid populations: maximizing data availability from many small samples. PLoS One 9:e102859
Tsiftsis S, Djordjević V (2020) Modelling sexually deceptive orchid species distributions under future climates: the importance of plant–pollinator interactions. Sci Rep-UK 10:1–12
Tylianakis JM, Didham RK, Bascompte J, Wardle DA (2008) Global change and species interactions in terrestrial ecosystems. Ecol Lett 11(12):1351–1363
Van der Pijl L, Dodson CH (1966) Orchid flowers: their pollination and evolution. University of Miami Press, Coral Gables
Van der Putten WH, Macel M, Visser ME (2010) Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos T Roy Soc B 365:2025–2034
Vila M, Pujadas J (2001) Land-use and socio-economic correlates of plant invasions in European and North African countries. Biol Conserv 100:397–401
Vitousek PM, Walker LR (1989) Biological invasion by Myrica faya in Hawai’i: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59:247–265
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin J, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389
Walther GR (2010) Community and ecosystem responses to recent climate change. Philos T Roy Soc B 365:2019–2024
Yeh C-M, Chung KM, Liang C-K, Tsai W-C (2019) New insights into the symbiotic relationship between orchids and fungi. Appl Sci 9:585
Zotz G (2016) Plants on plants - the biology of vascular epiphytes. Springer International Publishing, Switzerland
Acknowledgements
We thank Lydia Soifer for providing weevil waypoints and guidance with GIS and Maxent; Wilfredo Falcón for assistance with Maxent; and Steve Silvestrini for localities of remote populations.
Funding
Funding was provided by a grant from the National Science Foundation-Research. Experience for Undergraduates program (DBI-1930099, A. Ramírez, PI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no financial or non-financial conflicts of interest.
Consent for publication
All authors provide their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Foster, E.A., Ackerman, J.D. Future changes in the distribution of two non-indigenous orchids and their acquired enemy in Puerto Rico. Biol Invasions 23, 3545–3563 (2021). https://doi.org/10.1007/s10530-021-02596-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-021-02596-3