Abstract
Introduction
Since 2010, 10-valent (PCV10) and 13-valent pneumococcal conjugate vaccines (PCV13) have been available as part of infant national immunization programs. Belgium is as one of the few countries that implemented PCV13 (2007–2015), switched to PCV10 (2015–2018) and then switched back to PCV13 (2018–present) after observing increases in disease. We assessed the impacts of both historical and prospective PCV choice in the context of the Belgian health care system and used this experience to validate previously developed economic models.
Methods
Using historical incidence (2007–2018) of pneumococcal disease for Belgian children aged < 16 years, observed invasive pneumococcal disease (IPD) trends from surveillance data were used to estimate future disease in a given PCV13- or PCV10-based program. We compared observed incidence data with two modeled scenarios: (1) the 2015 switch to PCV10 and (2) a hypothetical continuation of PCV13 in 2015. Finally, we explored the potential impact of PCV choice from 2019 to 2023 by comparing three scenarios: (3) continued use of PCV10; (4) a switch back to PCV13; (5) a hypothetical scenario in which Belgium never switched from PCV13.
Results
Model predictions underestimated observed data from 2015 to 2018 by 100 IPD cases among ages < 16 years. Comparing observed data with scenario 2 suggests that PCV13 would have prevented 105 IPD cases from 2015 to 2018 compared with PCV10. Switching to PCV13 in 2019 would avert 625 IPD cases through 2023 compared with continuing PCV10. Scenario never switching from PCV13 would have resulted in a reduction of 204 cases from 2016 to 2023 compared with switching to PCV10 and switching back to PCV13.
Conclusion
The findings from this study suggest that previously published modeling results of PCV13 versus PCV10 in other countries may have underestimated the benefit of PCV13. These results highlight the importance of continually protecting against vaccine-preventable pneumococcal serotypes.
Similar content being viewed by others
Why carry out this study? |
Belgium switched from a 13-valent pneumococcal vaccine to a 10-valent vaccine in 2015, making it one of the few settings in which data generation for a switch from PCV13 is possible. |
The objectives of this study are to assess the impact of PCV13 compared with PCV10 in Belgium and to use the experience in Belgium to validate the findings of our previously developed health economic disease prediction models. |
What was learned from this study? |
The model underpredicted the re-emergence of 19A in Belgium following the switch to PCV10. |
Switching back to PCV13 is estimated to result in 625 fewer cases of IPD in children from 2019 to 2023. |
Never having switched to PCV10 in 2015 would have saved an additional 204 cases in addition to the 625 cases averted by switching back to PCV13 in 2019. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14785272.
Introduction
Streptococcus pneumoniae is a gram-positive bacterium with > 90 serotypes associated with invasive pneumococcal diseases (IPD), such as meningitis and septicemia, as well as non-invasive more common diseases, such as pneumonia and acute otitis media. Since the early 2000s, pneumococcal conjugate vaccines (PCVs) containing 7 (PCV7, Prevnar®, Wyeth Lederle Vaccines), 10 (PCV10, Synflorix®, GlaxoSmithKline Biologicals S.A.) and 13 serotypes (PCV13, Prevnar 13®, Wyeth/Pfizer Vaccines) have been developed and implemented across the world for use in routine infant national immunization programs (NIPs). Since their introduction, the use of PCVs globally has substantially reduced the burden of vaccine-type pneumococcal disease [14].
In Belgium, PCV7 was first introduced in 2007, in a two-plus-one schedule, resulting in a significant reduction in cases of IPD due to the PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F and 23F). The proportion of PCV7 serotypes decreased from 67% of IPD cases in 2003 to 7% of cases in 2008, which demonstrated successful suppression of vaccine-serotype disease [5]. However, the incidence of IPD due to serotype 19A increased during this time, representing 40% of all IPD cases by 2010 [2]. Subsequently, in 2011, Belgium replaced PCV7 with PCV13, which contains the same serotypes as PCV7 plus serotypes 1, 3, 5, 6A, 7F and 19A. This resulted in > 90% reduction in IPD cases caused by 19A in children < 2 years old from 25 cases per 100,000 during the PCV7 era to 2.2 cases per 100,000 in the PCV13 era. In 2015, the Belgian Superior Health Council stated that both 10-valent (PCV10) and 13-valent pneumococcal conjugate vaccine PCV13 meet the need to protect Belgian children against IPD [3]. Given the low levels of circulating vaccine-type disease, associated low levels of carriage due to PCV13 serotypes and cost of the infant PCV program, Flanders switched to PCV10 in July 2015, followed by Wallonia in May 2016 [2, 18].
Although PCV10 contains ten common serotypes compared with PCV13, it lacks serotypes 3, 6A and 19A. Following this transition, all-IPD incidence increased from 43.4 cases per 100,000 in children < 2 years during the 2015–2016 period to 58.4 cases per 100,000 in 2017–2018. Incidence of IPD cases caused by PCV13-only serotypes (3, 6A, and 19A) increased from 2.6 cases per 100,000 in 2016 to 20.9 cases per 100,000 in 2018. This trend was viewed concurrently in nasopharyngeal carriage data, where carriage prevalence of PCV13-only serotypes increased from 0.9% in 2016 to 7.8% in 2017/2018 with 19A being the most common isolated serotype [18]. Ultimately, given the robust surveillance data available in Belgium to identify changes in pneumococcal epidemiology, the Superior Health Council issued a preferred recommendation for PCV13 and to replace PCV10 with PCV13 in the immunization program in 2019 [4].
Previously, a decision-analytic model was developed to predict the clinical and economic benefit of maintaining use of PCV13 compared with switching to PCV10 in countries where PCV13 was being used as part of routine infant immunization [6, 8, 9, 10, 15, 17]. However, limited real-world evidence was available at the time of model development to validate the projections from those analyses. Given that Belgium is one of the first countries to change its NIP from PCV13 to PCV10, it is an ideal case study to assess the external validity of this model and to understand the opportunity cost of certain decisions that have occurred by looking at the impact of decisions over longer potential time periods.
The objectives of this study are to assess the impact of PCV13 compared with PCV10 in Belgium and to use the experience in Belgium to validate the findings of our previously developed economic models. Specifically, we explore three scenarios: (1) to compare the observed incidence from 2015 to 2018 with the model estimated incidence following the switch to PCV10 in 2015 as an assessment of previous models that estimated the cost-effectiveness of switching from PCV13 to PCV10 [15, 17], (2) to compare the observed impact of switching to PCV10 in 2015 compared with the estimated impact of maintaining PCV13 and (3) to estimate the potential impact of switching back to PCV13 in 2019 compared with maintaining PCV10.
Methods
Model Structure
We used a previously developed decision-analytic model (see Supplementary Material) to estimate the public clinical and economic impact of a switch from a PCV10-based infant vaccination program to a PCV13-based program in Belgium [17]. This model framework and assumptions have been detailed in previous publications [6, 8, 9, 10, 15, 17]. Briefly, we used observed IPD surveillance data by serotype from periods with and without serotype-specific vaccine coverage to project disease incidence with either PCV13 or PCV10.
Population and Comparators
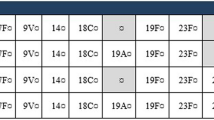
We modeled the population ages 0–15 years of Belgium (2,049,978), of which ~ 240,000 children < 2 years of age are eligible for pneumococcal vaccination annually (0). Ninety-four percent of infants were assumed to be vaccinated each year [16]. To account for age-specific differences in incidence, we stratified the population into the following age groups: 0 < 2 years, 2–4 years and 5–15 years. These age groups were selected to align with those used in the historical IPD data from Desmet et al. [2] (Table 1).
Estimating Invasive Pneumococcal Disease Cases
To forecast the number of IPD cases, historical, age- and serotype-specific surveillance data from 2007 to 2018 were obtained from Desmet et al. [2] as presented in 0. Serotype- and age-group-specific trendlines were then independently fit to the observed data as outlined in detail in a previous study [17]. Specifically, we estimated incidence trends based on the observed Belgian data. The projected IPD trend equations for each age group and serotype are presented in Supplemental Table 1. Because incidence data were not available prior to the introduction of PCVs in Belgium, we capped forecasted disease re-emergence to not exceed 125% of the incidence reported in 2007, which was the first year of available continuous data (see 0). In previous iterations of this model, a lag was used to account for a delay in re-emergence of non-vaccine serotypes [15, 17]. However, in this analysis we did not consider a lag given the rapid replacement of 19A observed in Belgium following the switch from PCV13 to PCV10 (Table 2).
Mortality
General population all-cause mortality was derived using demographics and number of deaths reported by Statbel [12]. Additional mortality estimates due to IPD for ages 0–15 years were obtained from Statbel [12] (0). These data were included to ensure we accounted for the correct population size in each year, reflective of deaths occurring in each age group from causes other than pneumococcal disease.
Calculations
We projected IPD incidence by age and serotype over the modeled time horizon and estimated number of IPD cases and IPD deaths incurred over the model time horizon for each treatment arm.
Comparisons Considered
To address the study objectives, we developed a series of scenarios. First, we examined the period from 2015 to 2018 (historical analysis) with a pair of scenarios compared with the observed incidence over the period. Additionally, we conducted three forward looking forecasted scenarios. These scenarios are outlined below.
In the first historical scenario (scenario 1), we estimated the anticipated effect of a switch from PCV13 to PCV10 in 2015. When compared with observed data, this scenario tests the robustness of our model assumptions and helps to determine whether the model methodology would have over- or underestimated the impact of switching from PCV13 to PCV10. This also serves as a validation of previous studies using this methodology in Mexico and Canada, for which we projected incidence for a hypothetical switch to PCV10 in the absence of observed data [15, 17]. In this scenario, the model’s projected incidence from 2016 to 2018 was estimated based on historic serotype trends from Finland for PCV10.
In the second scenario (scenario 2), we estimated a hypothetical projection of incidence had Belgium never switched to PCV10 and continued PCV13 use after 2015. This analysis allows an estimate of the overall impact on disease due to the switch to PCV10 when compared with the observed data. In this analysis, we consider data up to 2015 (at which point Belgium switched to PCV10) for PCV13 and estimate incidence with continued use of PCV13. We then compared these data to the observed data from 2016 to 2018 for the PCV10 scenario. As with the first analysis, the comparison was made over a 3-year period (2016–2018).
For the forecasted analysis, we projected incidence beginning in 2019 for three scenarios. First (in scenario 3), we estimated the expected incidence following the switch back to PCV13 from 2019 to 2023. Next (in scenario 4), we projected incidence for a setting in which Belgium continued use of PCV10 from 2019 to 2023. Finally (in scenario 5), we projected the incidence from 2019 to 2023 for a hypothetical scenario in which Belgium had never switched to PCV10 in 2015 (i.e., always used PCV13).
In summary, along with the observed historical IPD incidence in Belgium from 2015 to 2018, the five modeled scenarios are outlined in 0 and are as follows:
-
Scenario 1: Historical modeled incidence (2016–2018) for the switch to PCV10 in 2015.
-
Scenario 2: Historical modeled incidence (2016–2018) for a hypothetical scenario in which Belgium did not’ switch to PCV10 in 2015.
-
Scenario 3: Future projected incidence assuming Belgium continued use of PCV10 as of 2019 (2019–2023).
-
Scenario 4: Future projected incidence (2019–2023) for the switch back to PCV13 in 2019.
-
Scenario 5: Future projected incidence (2019–2023) for the hypothetical scenario in which Belgium never switched to PCV10, whereby projections of historical modeled incidence for PCV13 (2016–2018) in scenario 2 are continued.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors (Table 3).
Results
Historical Observed Versus Historical Modeled Invasive Pneumococcal Disease Incidence from 2015 to 2018
For scenarios 1 and 2, we found that the observed incidence exceeded both the PCV13 and PCV10 modeled projections for 2016–2018 time period (0). Overall observed IPD incidence in Belgium from 2015 to 2018 for children < 16 years increased by nearly 4 cases per 100,000 from 2015 to 2018 while using PCV10. By comparison, scenario 1’s forecasted projections, which modeled the impact of PCV10 introduction in 2015/2016, only saw a modest increase in incidence. The discrepancy between the observed historical data and our projected scenario 1 was driven primarily by an increase in incidence in those aged < 2 years, with serotype 19A incidence representing most of the increase. Therefore, the model projections underestimated the true observed increase in disease. In scenario 2, which modeled the continued use of PCV13, estimated projections saw a decrease in incidence and sustained reductions in serotype 19A compared with the observed incidence over 2016–2018 (Table 4).
Future Projected Invasive Pneumococcal Disease Incidence for 2019–2023 Under PCV10 or PCV13 Programs
In the forecasted analysis from 2019 to 2023, we found that re-introducing PCV13 in 2019 (scenario 4) is expected to substantially reduce disease incidence compared with continuing use of PCV10 (scenario 3) (0). For scenario 3, the increase in incidence was driven by the continued re-emergence of serotype 19A as was observed from 2015 to 2018, along with a smaller increase in serotype 3 and non-PCV10 type disease. For scenario 4, non-PCV13 type and serotype 3 represented most of the cases (Table 5).
Future Projected Invasive Pneumococcal Disease Incidence from 2019 to 2023 After Modeled Use of PCV13 Throughout 2015–2018
A last scenario considering a hypothetical case in which Belgium never switched to PCV10 (scenario 5) resulted in the lowest incidence of the three prospective scenarios from 2019 to 2023. This was driven by a substantial reduction in incidence from 2016 to 2018 compared with the observed data, which resulted in a starting incidence of 9.39 per 100,000 for scenario 5 compared with 13.53 for scenarios 3 and 4. In this scenario, the increase in incidence over time was driven almost entirely by non-PCV13 type disease.
Case and Death Outcomes for Historical Observed (2016–2018), Historical Modeled (2016–2018) and Future Model (2019–2023) Scenarios
Based on the incidence data presented in 0, we estimated the total cases and IPD deaths each year for each scenario (Fig. 1). The model estimated 575 cases and 25 deaths from IPD in children < 16 years for scenario 1 (switching to PCV10 as of 2015/16) from 2016 to 2018. Compared with the 675 cases and 29 deaths observed in Belgium, our model underestimated the actual disease replacement by approximately 100 IPD cases and 4 IPD related deaths. Similarly, the model estimated that maintaining use of PCV13 as of 2015 (scenario 2) would have resulted in 105 fewer IPD cases (570 total) and 5 fewer IPD deaths (24 total) compared with the observed data.
In the forecasted analysis, the model found that switching back to PCV13 in 2019 (scenario 4) was expected to result in 625 fewer cases of IPD and 12 fewer IPD deaths compared with continuing use of PCV10 over the next 5 years (scenario 3) (Table 6). Had Belgium never switched to PCV10 (scenario 5), the continued use of PCV13 would have resulted in an estimated 204 fewer IPD cases and 8 fewer deaths due to IPD in children ages < 16 years from 2016 to 2023 (105 fewer cases from 2016 to 2018 and an additional 99 fewer cases from 2019 to 2023).
Discussion
We previously developed a decision-analytic model to assess the public health and economic impact of switching PCVs on pediatric NIPs by leveraging historical observed epidemiologic surveillance data. We sought to use the PCV switching experience and epidemiologic data in Belgium as a case study to validate this previously developed model and its findings. Therefore, this study sought to accomplish the following objectives: (1) to estimate the epidemiologic impact of the switch to PCV10 compared with never having switched from PCV13; (2) to validate the findings of previous research into the hypothetical switch to PCV10 in PCV13 countries; (3) to estimate the potential impact of sustained use of PCV10 compared with switching back to PCV13 in Belgium.
Each of the scenario analyses developed using the comparison of PCV13 and PCV10 in Belgium provides different insights to meet our study’s objective. In scenario 1, we modeled PCV10 from 2016 to 2018 assuming trendline equations based on Belgium data from 2007 to 2015. Comparing our modeled historical scenario 1 to real-world historical observed data, we find that the observed Belgium data as of 2015 substantially underestimated overall incidence and most notably underestimated 19A re-emergence once vaccine pressure on this serotype was removed, despite 19A trendlines historically fitting well to observed surveillance trends with and without vaccine pressure. This is an important finding because it suggests that significant reductions in vaccine serotypes in IPD may not mean circulation of carriage has been controlled, and other countries that have used this model to estimate a switch from PCV13 to PCV10 may underestimate the benefits of maintaining a PCV13 program. The switch to PCV10 resulted in a nearly 4% increase in cases of disease over the 3-year period during which PCV10 was used. Although we estimated only a small benefit of PCV13 compared with PCV10 over the 3-year period, the observed increase in incidence of serotype 19A was much greater than the model predicted. Analyses conducted for Canada [17] and Mexico [15] suggested that maintaining the use of PCV13 would be cost saving, and, as such, an underestimate of the increase in 19A would merely strengthen the public health and economic advantage of PCV13. In one recent analysis for The Netherlands, PCV13 was considered cost-effective, not cost saving compared with PCV10 [9]; however, this analysis was based on the limited 19A replacement that has uniquely been seen in The Netherlands in contrast to other countries using PCV10. We can compare an analogous analysis for Belgium using scenarios 3 and 4 where the future modeled incidence for PCV10 or PCV13 compares the potential benefit of switching back to PCV13 versus a scenario continuing the use of PCV10. In this comparison, we found that switching back to PCV13 should lead to a substantial reduction in disease burden over the next 5 years. Finally, scenario 5 compared with scenarios 3 and 4 illustrates the sustained impact in 2019–2023 resulting from the switch in 2015. We found that maintaining PCV13 on the NIP and never switching to PCV10 would not only have resulted in fewer cases from 2016 to 2018, but the reduction in incidence over that period would have also led to further reductions in cases well into the future.
Based on historical data in Belgium, the model findings for the historical observed and modeled comparisons suggest that projecting disease re-emergence is challenging, especially given the perceived exceptionally low levels of circulating vaccine-type disease. Specifically, the model substantially underestimated the re-emergence of 19A. In fact, our model would have supported Belgium’s economic decision in 2015, as incidence of 19A was low and the modeled historical predictions anticipated a slower re-emergence of disease. However, 19A incidence re-emerged more exponentially than anticipated, and, as such, Belgium made a proactive recommendation to switch back to PCV13 after seeing a nearly 40% re-emergence after only 2 years of PCV10 use. The Belgian experience may serve as a useful illustration of the limitations of modeling disease re-emergence in the absence of prior evidence for previously covered vaccine serotypes. For serotypes that had previously shown relatively high prevalence but for which PCV serotype coverage has largely eradicated incidence, the experience of 19A in Belgium from 2015 to 2018 suggests vaccine-type disease re-emergence may be more substantial than modeling exercises predict. This differs from scenarios in which a novel vaccine is introduced (such as the switch to a higher valent vaccine) and, perhaps, also when compared with removing vaccine coverage of a serotype that has not been as prevalent prior to the introduction of PCVs. Higher valent vaccines in development will contain serotypes identified as important causes of disease or antibiotic resistance. In some settings, these serotypes are acting as replacement serotypes and are increasing in incidence in both adult and pediatric populations; thus far, none of the serotypes express the propensity for carriage nor the invasiveness (case:carrier ratio) as has been observed with 19A.
It is important to note that the findings here should be considered conservative with respect to the full impact in the Belgian population. First, we did not consider any impact of infant vaccination on adult incidence in this study due to limited availability of pneumococcal incidence data for adults in Belgium. Worldwide, there is widespread and consistent evidence of indirect protection from infant PCV vaccination; given the observations seen in nasopharyngeal carriage data in Belgium following the switch to PCV10, it is likely that there would be broader impact of this change in vaccine policy than just observed in the younger population [11, 18]. Inclusion of adult incidence in this Belgian analysis thus would presumably lead to a larger benefit from PCV13 use, and this indirect impact would come at no additional vaccination cost to the Belgian government.
As with any modeling exercise, our approach is subject to limitations. A key assumption is that historical serotype trends will inform future disease incidence. Notably, our approach may under- or overestimate the impact of disease re-emergence (as we see here with 19A), and it may under- or overestimate replacement of other serotypes after a change in vaccine program. Given that we rely on historical data, we might be unable to capture the emergence of a new serotype or group of serotypes that may replace incidence of the 13 covered serotypes. We have not seen any serotype emerge like 19A following the introduction of PCV7, and so the concern may be somewhat mitigated by the absence of a newly dominant serotype. Finally, as in all pneumococcal vaccine economic analyses, this study is also subject to the limitation of available data. Specifically, we do not have serotype-specific information for all of Belgium, nor do we have complete information for several other model parameters. It is unclear what impact these uncertainties would have on results.
This study adds to the body of evidence surrounding the impact of PCVs and is the first analysis to estimate the epidemiologic impact of switching back to PCV13 using observed surveillance data from a country that had previously switched from the 10- to the 13-valent vaccine pneumococcal vaccine. The findings further suggest the benefit of PCV13 over PCV10 and illustrate that disease re-emergence is an important factor when considering switching to a lower-valent vaccine. Based on the experience in Belgium, serotypes that were relatively high in prevalence before introduction of a vaccine may be likely to re-emerge more rapidly. As a result, cost savings from a less expensive vaccine program may be quickly eroded by an increase in medical costs due to increases in disease incidence.
Conclusions
In conclusion, this study validates a previously established PCV forecasting model to determine the future impact of a change in infant PCV policy and validates the decision-making process followed by the Belgium Health Council to switch back to PCV13. Notably, this analysis underscores the uncertainty surrounding serotype replacement, but it highlights the need to maximize coverage of highly invasive serotypes, specifically those that have been widely circulating prior to the use of higher valent PCVs. This will be of utmost importance as lower-valent and higher valent PCVs become available in future years.
References
Beutels P, Blommaert A, Hanquet G, Bilcke J, Thiry N, Sabbe M, et al. Cost-effectiveness of 10- and 13-valent pneumococcal conjugate vaccines in childhood. Health Technology Assessment (HTA). Brussels: Health Care Knowledge Centre (KCE) Report 155C; 2011.
Desmet S, Lagrou K, Wyndham-Thomas C, Braeye T, Verhaegen J, Maes P, et al. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. 2021;21(1):127–36.
Gezondheidsraad H. Advies van de Hoge Gezondheidsraad nr. 8813. Vaccinatie van kinderen en adolescenten tegen pneumokokken. 2015. https://www.health.belgium.be/nl/advies8813-vaccinatie-pneumokokken-kinderen-fiche. Accessed 13 Jan 2021.
Gezondheidsraad H. Vaccinatie tegen pneumokokken - kinderen en adolescenten (HGR 9519) 2018. https://www.health.belgium.be/en/node/34691#anchor-34691. Accessed 13 Jan 2021.
Hanquet G, Lernout T, Vergison A, Verhaegen J, Kissling E, Tuerlinckx D, et al. Impact of conjugate 7-valent vaccination in Belgium: addressing methodological challenges. Vaccine. 2011;29(16):2856–64.
Kim HY, Park SB, Kang ES, Lee SM, Kim HJ, Wasserman M. Cost-effectiveness of a national immunization program with the 13-valent pneumococcal conjugate vaccine compared with the 10-valent pneumococcal conjugate vaccine in South Korea. Hum Vaccines Immunother. 2020;17(3):1–10.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14.
Perdrizet J, Santana CFS, Senna T, Alexandre RF, Sini de Almeida R, Spinardi J, et al. Cost-effectiveness analysis of replacing the 10-valent pneumococcal conjugate vaccine (PCV10) with the 13-valent pneumococcal conjugate vaccine (PCV13) in Brazil infants. Hum Vaccines Immunother. 2021;17(4):1162–72.
Pugh S, Wasserman M, Moffatt M, Marques S, Reyes JM, Prieto VA, et al. Estimating the impact of switching from a lower to higher valent pneumococcal conjugate vaccine in Colombia, Finland, and The Netherlands: a cost-effectiveness analysis. Infect Dis Ther. 2020;9(2):305–24.
Shafie AA, Ahmad N, Naidoo J, Foo CY, Wong C, Pugh S, et al. Estimating the population health and economic impacts of introducing a pneumococcal conjugate vaccine in Malaysia- an economic evaluation. Hum Vaccines Immunother. 2020;16(7):1719–27.
Shiri T, Datta S, Madan J, Tsertsvadze A, Royle P, Keeling MJ, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–9.
Statbel. Directorate-General Statistics - Statistics Belgium. Mortality, Life expectancy and causes of death. 2020a. https://statbel.fgov.be/en/themes/population/mortality-life-expectancy-and-causes-death. Accessed 26 Oct 2020.
Statbel. Directorate-General Statistics - Statistics Belgium. Population by place of residence, nationality (Belgian/non-Belgian), marital status, age and gender. 2020b. https://bestat.statbel.fgov.be/bestat/crosstable.xhtml?view=e35b8fd3-ac11-4de9-b35b-3f3d1cf8399a. Accessed 19 Oct 2020.
Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–57.
Wasserman M, Palacios MG, Grajales AG, Baez/Revueltas FB, Wilson M, McDade C, et al. Modeling the sustained use of the 13-valent pneumococcal conjugate vaccine compared to switching to the 10-valent vaccine in Mexico. Hum Vaccines Immunother. 2019;15(3):560–69.
WHO. World Health Organization, immunization, vaccines and biologicals data, statistics and graphics. 2021. http://www.who.int/entity/immunization/monitoring_surveillance/data/coverage_estimates_series.xls. Accessed 13 Jan 2021.
Wilson M, Wasserman M, Jadavi T, Postma M, Breton MC, Peloquin F, et al. Clinical and economic impact of a potential switch from 13-valent to 10-valent pneumococcal conjugate infant vaccination in Canada. Infect Dis Ther. 2018;7(3):353–71.
Wouters I, Desmet S, Van Heirstraeten L, Herzog SA, Beutels P, Verhaegen J, et al. How nasopharyngeal pneumococcal carriage evolved during and after a PCV13-to-PCV10 vaccination programme switch in Belgium, 2016 to 2018. Euro Surveill. 2020;25(5):32–44.
Acknowledgements
Funding
This study was conducted by RTI Health Solutions, Research Triangle Park, NC, under the direction of Pfizer, Inc., and was funded by Pfizer, Inc., New York, NY. Pfizer, Inc. provided funding for the journal’s Rapid Service fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship contributions
Michele R. Wilson, Annick Mignon, Raymond A. Farkouh and Matt D. Wasserman contributed to concept of the work. Michele R. Wilson, Cheryl McDade, Johnna E. Perdrizet, Annick Mignon, Raymond A. Farkouh and Matt D. Wasserman contributed to statistical analysis, drafting, reviewing and revising the manuscript.
Disclosures
Michele R. Wilson, and Cheryl L. McDade received consulting fees from Pfizer Inc. as part of this research. Johnna E. Perdrizet, Raymond A. Farkouh and Matt D. Wasserman are employees of Pfizer Inc. Annick Mignon is an employee of Pfizer SA/NV, Belgium.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the author.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary material.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wilson, M.R., McDade, C.L., Perdrizet, J.E. et al. Validation of a Novel Forecasting Method for Estimating the Impact of Switching Pneumococcal Conjugate Programs: Evidence from Belgium. Infect Dis Ther 10, 1765–1778 (2021). https://doi.org/10.1007/s40121-021-00485-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00485-9