Abstract
Background
Multiple organ failure (MOF) is the main cause of early death in septic shock. Lungs are among the organs that are affected in MOF, resulting in acute lung injury. Inflammation is an important factor that causes immune cell dysfunction in the pathogenesis of sepsis. Autophagy is involved in the process of inflammation and also occurs in response to cell and tissue injury in several diseases. We previously demonstrated that hydrogen alleviated the inflammation-induced cell injury and organ damage in septic mice.
Aim
The focus of the present study was to elucidate whether mitophagy mediates the inflammatory response or oxidative injury in sepsis in vitro and in vivo. Furthermore, we evaluated the role of mitophagy in the protective effects of hydrogen against cell injury or organ dysfunction in sepsis.
Method
RAW 264.7 macrophages induced by lipopolysaccharide (LPS) were used as an in vitro model for inflammation, and cecal ligation and puncture (CLP)-induced acute lung injury mice were used as an in vivo model for sepsis. The key protein associated with mitophagy, PTEN-induced putative kinase 1 (PINK1), was knocked down by PINK1 shRNA transfection in RAW 264.7 macrophages or mice.
Results
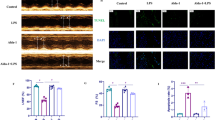
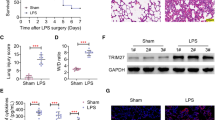
Hydrogen ameliorated cell injury and enhanced mitophagy in macrophages stimulated by LPS. PINK1 was required for the mitigation of the cell impairment in LPS-stimulated macrophages by hydrogen treatment. PINK1 knockdown abrogated the beneficial effects of hydrogen on mitophagy in LPS-stimulated macrophages. Hydrogen inhibited acute lung injury in CLP mice via activation of PINK1-mediated mitophagy.
Conclusion
These results suggest that PINK1-mediated mitophagy plays a key role in the protective effects of hydrogen against cell injury in LPS-induced inflammation and CLP-induced acute lung injury.










Similar content being viewed by others
References
Rim TH, Han JS, Oh J, Kim DW, Kang SM, Chung EJ. Retinal vein occlusion and the risk of acute myocardial infarction development: a 12-year nationwide cohort study. Sci Rep. 2016;6:22351.
Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, Nagarkatti M. Role of cytokines as a double-edged sword in sepsis. Vivo. 2013;27:669–84.
Gao R, Ma Z, Hu Y, Chen J, Shetty S, Fu J. Sirt1 restrains lung inflammasome activation in a murine model of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L847-853.
Varisco BM. The pharmacology of acute lung injury in sepsis. Adv Pharmacol Sci. 2011;2011:254619.
Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64.
Deng Q, Zhao T, Pan B, Dennahy IS, Duan X, Williams AM, Liu B, Lin N, Bhatti UF, Chen E, Alam HB, Li Y. Protective effect of tubastatin A in CLP-induced lethal sepsis. Inflammation. 2018;41:2101–9.
Slavin SA, Leonard A, Grose V, Fazal F, Rahman A. Autophagy inhibitor 3-methyladenine protects against endothelial cell barrier dysfunction in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2018;314:L388-396.
Sun DZ, Song CQ, Xu YM, Wang R, Liu W, Liu Z, Dong XS. Involvement of PINK1/Parkin-mediated mitophagy in paraquat- induced apoptosis in human lung epithelial-like A549 cells. Toxicol In Vitro. 2018;53:148–59.
Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS. Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 2018;138:2247–62.
Aguirre A, Lopez-Alonso I, Gonzalez-Lopez A, Amado-Rodriguez L, Batalla-Solis E, Astudillo A, Blazquez-Prieto J, Fernandez AF, Galvan JA, Dos SC, Albaiceta GM. Defective autophagy impairs ATF3 activity and worsens lung injury during endotoxemia. J Mol Med (Berl). 2014;92:665–76.
Colell A, Ricci JE, Tait S, Milasta S, Maurer U, Bouchier-Hayes L, Fitzgerald P, Guio-Carrion A, Waterhouse NJ, Li CW, Mari B, Barbry P, Newmeyer DD, Beere HM, Green DR. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–97.
Lo S, Yuan SS, Hsu C, Cheng YJ, Chang YF, Hsueh HW, Lee PH, Hsieh YC. Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann Surg. 2013;257:352–63.
Mizumura K, Cloonan S, Choi ME, Hashimoto S, Nakahira K, Ryter SW, Choi AM. Autophagy: friend or foe in lung disease? Ann Am Thorac Soc. 2016;13(Suppl 1):S40–7.
Ghavami S, Yeganeh B, Zeki AA, Shojaei S, Kenyon NJ, Ott S, Samali A, Patterson J, Alizadeh J, Moghadam AR, Dixon IMC, Unruh H, Knight DA, Post M, Klonisch T, Halayko AJ. Autophagy and the unfolded protein response promote profibrotic effects of TGF-β in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2018;314:L493-504.
Takaoka Y, Goto S, Nakano T, Tseng HP, Yang SM, Kawamoto S, Ono K, Chen CL. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) prevents lipopolysaccharide (LPS)-induced, sepsis-related severe acute lung injury in mice. Sci Rep. 2014;4:5204.
Hammerling BC, Gustafsson AB. Mitochondrial quality control in the myocardium: cooperation between protein degradation and mitophagy. J Mol Cell Cardiol. 2014;75:122–30.
Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–21.
Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–73.
West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402.
Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23.
Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–51.
Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298.
Chen HR, Chuang YC, Chao CH, Yeh TM. Macrophage migration inhibitory factor induces vascular leakage via autophagy. Biol Open. 2015;4:244–52.
Chen H, Xie K, Han H, Li Y, Liu L, Yang T, Yu Y. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int Immunopharmacol. 2015;28:643–54.
Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, Wang G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34:90–7.
Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong L, Hou L, Wang G. Hydrogen gas improves survival rate and organ damage in zymosan-induced generalized inflammation model. Shock. 2010;34:495–501.
Dong A, Yu Y, Wang Y, Li C, Chen H, Bian Y, Zhang P, Zhao Y, Yu Y, Xie K. Protective effects of hydrogen gas against sepsis-induced acute lung injury via regulation of mitochondrial function and dynamics. Int Immunopharmacol. 2018;65:366–72.
Zhang X, Yuan D, Sun Q, Xu L, Lee E, Lewis AJ, Zuckerbraun BS, Rosengart MR. Calcium/calmodulin-dependent protein kinase regulates the PINK1/Parkin and DJ-1 pathways of mitophagy during sepsis. FASEB J. 2017;31:4382–95.
Chen H, Hu Y, Xie K, Chen Y, Wang H, Bian Y, Wang Y, Dong A, Yu Y. Effect of autophagy on allodynia, hyperalgesia and astrocyte activation in a rat model of neuropathic pain. Int J Mol Med. 2018;42:2009–19.
Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K. Inhalation of hydrogen gas reduces infarct size in therat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373(1):30–5. https://doi.org/10.1016/j.bbrc.2008.05.165.
Qiu BY, Turner N, Li YY, Gu M, Huang MW, Wu F, Pang T, Nan FJ, Ye JM, Li JY, Li J. High-throughput assay for modulators of mitochondrial membrane potential identifies a novel compound with beneficial effects on db/db mice. Diabetes. 2010;59:256–65.
Zhao C, Chen Z, Xu X, An X, Duan S, Huang Z, Zhang C, Wu L, Zhang B, Zhang A, Xing C, Yuan Y. Pink1/Parkin-mediated mitophagy play a protective role in cisplatin induced renal tubular epithelial cells injury. Exp Cell Res. 2017;350:390–7.
Kang R, Zeng L, Xie Y, Yan Z, Zhou B, Cao L, Klionsky DJ, Tracey KJ, Li J, Wang H, Billiar TR, Jiang J, Tang D. A novel PINK1- and PARK2-dependent protective neuroimmune pathway in lethal sepsis. Autophagy. 2016;12:2374–85.
Lewis AJ, Billiar TR, Rosengart MR. Biology and metabolism of sepsis: innate immunity, bioenergetics, and autophagy. Surg Infect (Larchmt). 2016;17:286–93.
Li S, Wu H, Han D, Ma S, Fan W, Wang Y, Zhang R, Fan M, Huang Y, Fu X, Cao F. A novel mechanism of mesenchymal stromal cell-mediated protection against sepsis: restricting inflammasome activation in macrophages by increasing mitophagy and decreasing mitochondrial ROS. Oxid Med Cell Longev. 2018;2018:3537609.
Chen HG, Xie KL, Han HZ, Wang WN, Liu DQ, Wang GL, Yu YH. Heme oxygenase-1 mediates the anti-inflammatory effect of molecular hydrogen in LPS-stimulated RAW 264.7 macrophages. Int J Surg. 2013;11:1060–6.
Xie K, Fu W, Xing W, Li A, Chen H, Han H, Yu Y, Wang G. Combination therapy with molecular hydrogen and hyperoxia in a murine model of polymicrobial sepsis. Shock. 2012;38:656–63.
Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35.
Gordon S, Pluddemann A. Tissue macrophages: heterogeneity and functions. BMC Biol. 2017;15:53.
Luan YY, Dong N, Xie M, Xiao XZ, Yao YM. The significance and regulatory mechanisms of innate immune cells in the development of sepsis. J Interferon Cytokine Res. 2014;34:2–15.
Hamidzadeh K, Christensen SM, Dalby E, Chandrasekaran P, Mosser DM. Macrophages and the recovery from acute and chronic inflammation. Annu Rev Physiol. 2017;79:567–92.
Lauvau G, Loke P, Hohl TM. Monocyte-mediated defense against bacteria, fungi, and parasites. Semin Immunol. 2015;27:397–409.
Wang TS, Deng JC. Molecular and cellular aspects of sepsis-induced immunosuppression. J Mol Med (Berl). 2008;86:495–506.
Winkler MS, Rissiek A, Priefler M, Schwedhelm E, Robbe L, Bauer A, Zahrte C, Zoellner C, Kluge S, Nierhaus A. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFalpha response: a diagnostic tool for immunosuppression? PLoS ONE. 2017;12:e0182427.
Qiu P, Liu Y, Zhang J. Review: the role and mechanisms of macrophage autophagy in sepsis. Inflammation. 2018;42:6–19.
Deretic V, Kimura T, Timmins G, Moseley P, Chauhan S, Mandell M. Immunologic manifestations of autophagy. J Clin Invest. 2015;125:75–84.
Hsieh CH, Pai PY, Hsueh HW, Yuan SS, Hsieh YC. Complete induction of autophagy is essential for cardioprotection in sepsis. Ann Surg. 2011;253:1190–200.
Li F, Lang F, Zhang H, Xu L, Wang Y, Hao E. Role of TFEB mediated autophagy, oxidative stress, inflammation, and cell death in endotoxin induced myocardial toxicity of young and aged mice. Oxid Med Cell Longev. 2016;2016:5380319.
Ren J, Xu X, Wang Q, Ren SY, Dong M, Zhang Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J Mol Cell Cardiol. 2016;93:18–31.
Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–73.
Nardin A, Schrepfer E, Ziviani E. Counteracting PINK/Parkin deficiency in the activation of mitophagy: a potential therapeutic intervention for Parkinson’s disease. Curr Neuropharmacol. 2016;14:250–9.
Barodia SK, Creed RB, Goldberg MS. Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res Bull. 2017;133:51–9.
Mannam P, Shinn AS, Srivastava A, Neamu RF, Walker WE, Bohanon M, Merkel J, Kang MJ, Dela CC, Ahasic AM, Pisani MA, Trentalange M, West AP, Shadel GS, Elias JA, Lee PJ. MKK3 regulates mitochondrial biogenesis and mitophagy in sepsis-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;306:L604–19.
Zhang Y, Sauler M, Shinn AS, Gong H, Haslip M, Shan P, Mannam P, Lee PJ. Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J Immunol. 2014;192:5296–304.
Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, Youle RJ. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018;561:258–62.
Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway: a mitochondrial quality control system? J Bioenerg Biomembr. 2009;41:499–503.
Du F, Yu Q, Yan S, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, Yan SS. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain. 2017;140:3233–51.
Yunfu W, Guangjian L, Ping Z, Yanpeng S, Xiaoxia F, Wei H, Jiang Y, Jingquan H, Songlin W, Hongyan Z, Yong L, Shi C. PINK1 and its familial Parkinson’s disease-associated mutation regulate brain vascular endothelial inflammation. J Mol Neurosci. 2014;53:109–16.
Funding
This work was supported in part by National Natural Science Foundation of China (Nos. 81601667 to Hongguang Chen, 81671888 to Yonghao Yu, 81772043 to Xie Keliang, 81971879) Beijing, China; Natural Science Foundation of Tianjin (Nos. 18JCYBJC93700 to Hongguang Chen).
Author information
Authors and Affiliations
Contributions
YY and KX designed the research and draft the manuscript; HC performed the animal research; BD cultures cell and collected sample in vitro; HL and YW carried out the detection of indicators. HL revised the manuscript after major revision. YY performed the results statistic. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Tianjin Medical University and were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and the number of animals used.
Additional information
Responsible Editor: Anatoliy Kubyshkin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Lin, H., Dong, B. et al. Hydrogen alleviates cell damage and acute lung injury in sepsis via PINK1/Parkin-mediated mitophagy. Inflamm. Res. 70, 915–930 (2021). https://doi.org/10.1007/s00011-021-01481-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01481-y