Abstract
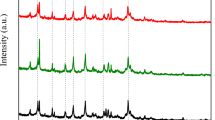
Samples of Na, Li, K, and Ca forms of dispersed LSX and Х zeolites of high phase purity and almost 100% degree of crystallinity were synthesized. The influence of the kind and content of the exchangeable cation on the adsorption characteristics of the porous structure of the synthesized cation-exchange forms of LSX and Х zeolites with respect to water and benzene vapors was studied. The amounts of the carbon dioxide adsorbed at 25°C from gas–air mixtures containing 0.04 and 10 vol % CO2 onto various ion-exchange forms of LSX and X zeolites until reaching the limiting capacity for СО2 were compared.






Similar content being viewed by others
REFERENCES
Ruthven, D.M., Farooq, S., and Knaebel, K., Pressure Swing Adsorption, New York: VCH, 1994.
Glupanov, V.N., Shumyatskii, Yu.I., Seregin, Yu.A., and Brekhner, S.A., Poluchenie kisloroda i azota adsorbtsionnym razdeleniem vozdukha (Production of Oxygen and Nitrogen by Adsorption Separation of Air), TsINTIKhimneftemash, 1991.
Mel’gunov, M.S., Korotkotsiklovaya beznagrevnaya adsorbtsiya (Pressure Swing Adsorption), Moscow: Kalvis, 2009.
Shumyatskii, Yu.I., Promyshlennye adsorbtsionnye protsessy (Industrial Adsorption Processes), Moscow: KolosS, 2009.
Bonenfant, D., Kharoune, M., Niquette, P., Mimeault, M., and Hausler, R., Sci. Technol. Adv. Mater., 2008, vol. 9, pp. 1–7. https://doi.org/10.1088/1468-6996/9/1/013007
Baciocchi, R., Storti, G., and Mazzotti, M., Chem. Eng. Process., 2006, vol. 45, pp. 1047–1058. https://doi.org/10.1016/j.cep.2006.03.015
Goeppert, A., Czaun, M., Prahash, G.K., and Olah, G.A., Energ. Environ. Sci., 2012, vol. 5, pp. 7833–7853. https://doi.org/10.1039/C2EE21586A
Cheng, H. and Tan, C., Sep. Purif. Technol., 2011, vol. 82, pp. 156–166. https://doi.org/10.1016/j.seppur.2011.09.004
Keith, D.W., Science, 2009, vol. 25, pp. 1654–1655. https://doi.org/10.1126/science.1175680
Breck, D.W., Zeolite Molecular Sieves: Structure, Chemistry and Use, New York: Wiley, 1974.
Pavlova, I.N., Travkina, O.S., and Garieva, G.F., Petrol. Chem., 2020, vol. 60, no. 8, pp. 903–908. https://doi.org/10.1134/S0965544120080101
Pavlova, I.N., Garieva, G.F., Travkina, O.S., Kutepov, B.I., Fomkin, A.A., and Shkolin, A.V., Prot. Met. Phys. Chem. Surf., 2015, vol. 51, no. 5, pp. 767–772.
Kel’tsev, N.V., Osnovy adsorbtsionnoi tekhniki (Principles of Adsorption Technique), Moscow: Khimiya, 1984.
Pavlova, I.N., Ilibaev, R.S., Travkina, O.S., Il’yasova, R.R., and Kutepov, B.I., Khim. Tekhnol., 2010, no. 4, pp. 208–213.
Kuronen, M., Weller, M., Townsend, R., and Harjula, R., React. Funct. Polym., 2006, vol. 66, no. 11, pp. 1350–1361. https://doi.org/10.1016/j.reactfunctpolym.2006.03.019
Treacy, M.M.J. and Higgins, J.B., Collection of Simulated XRD Powder Patterns for Zeolites, Elsevier, 2001.
Zhdanov, S.P., Khvoshchev, S.S., and Samulevich, N.N., Sinteticheskie tseolity. Kristallizatsiya, strukturnokhimicheskoe modifitsirovanie i adsorbtsionnye svoistva (Synthetic Zeolites. Crystallization, Structure-Chemical Modification, and Adsorption Properties), Moscow: Khimiya, 1981.
Hutson, N.D. and Yang, R.T., AIChE J., 2000, vol. 46, no. 11, pp. 2305–2317. https://doi.org/10.1002/aic.690461121
Jacobs, P.A., Van Cauwelaert, F.H., Vansant, E.F., and Uytterhoeven, J.B., J. Chem. Soc., Faraday Trans. 1, 1973, vol. 69, pp. 1056–1068. https://doi.org/10.1039/F19736901056
Montanari, T. and Busca, G., Vibr. Spectrosc., 2008, vol. 46, pp. 45–51. https://doi.org/10.1016/j.vibspec.2007.09.001
Hasegawa, K. and Matsumoto, A., AIP Conf. Proc., 2017, vol. 1865, 020002. https://doi.org/10.1063/1.4993321
Funding
The study was performed within the framework of the government assignment for the Institute of Petroleum Chemistry and Catalysis, Ufa Federal Research Center, Russian Academy of Sciences, theme: Zeolite Materials of Various Structural Types with High Degree of Crystallinity and Hierarchic Pore Structure: A New Generation of Practically Important Petrochemical Products, state registry no. AAAA-A19-119022290006-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Rights and permissions
About this article
Cite this article
Pavlova, I.N., Travkina, O.S., Garieva, G.F. et al. Activity of Various Cation-Exchange Forms of LSX and X Zeolites in СО2 Adsorption. Pet. Chem. 61, 925–931 (2021). https://doi.org/10.1134/S0965544121080065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544121080065