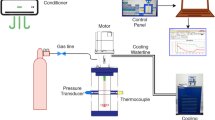
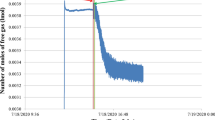
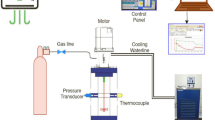
Abstract
The motivation for this study is the potential of environmental friendly amino acids such as alanine, histidine, phenylalanine and valine to be examined as inhibitors on methane (95%)—propane (5%) hydrate formation together with the hydrodynamic examination of three different flows in hydrate formation process. The outcomes indicated that valine and alanine behave as promoters while histidine and phenylalanine behave as inhibitors. From hydrodynamic perception radial flow shows less induction time values and better rate of hydrate formation due to better gas liquid contact compared to mixed flow experiments.







Similar content being viewed by others
References
Ripmeester JA, Tse JS, Ratcliffe CI, Powell BM (1987) A new clathrates hydrate structure. Nature 325:135–136
Sum AK, Burruss RC, Sloan ED (1997) Measurement of clathrate hydrates via Raman spectroscopy. J Phys Chem B 101:7371–7377
Merey S, Longinos SN (2018) Investigation of gas seepages in Thessaloniki mud volcano in the Mediterranean Sea. J Petrol Sci Eng 168:81–97. https://doi.org/10.1016/j.petrol.2018.05.014
Shahnazar S, Hasan N (2014) Gas hydrate formation condition: review on experimental and modeling approaches. Fluid Phase Equilib 379:72–85
Ansari F, Soofivand F, Salavati-Niasari M (2015) Utilizing maleic acid as a novel fuel for synthesis of PbFe12O19 nanoceramics via sol–gel auto-combustion route. Mater Charact 103:11–17
Carson DB, Katz DL (1942) Natural gas hydrates. Trans AIME 146:150–158
Kelland MA, Reyes FT, Trovik KW (2013) Tris (diakyloamino) cyclopropenium chlorides: tetrahydrofuran hydrate crystal growth inhibition and synergism with polyvinylcaprolactam as gas hydrate kinetic inhibitor. Chem Eng Sci 93:423–428
Sloan ED, Koh C (2007) Clathrate hydrates of natural gases, 3rd edn. Taylor & Francis, London
Cha M, Shin K, Kim J, Chang D, Seo Y, Lee H, Kang SP (2013) Thermodynamic and kinetic hydrate inhibition performance of aqueous ethylene glycol solutions for natural gas. Chem Eng Sci 99:184–190
Koh CA, Sloan ED, Sum AK, Wu DT (2011) Fundamentals and applications of gas hydrates. Annu Rev Chem Biomol Eng 2:237–257
Broni-Bediako E, Amorin R, Bavoh CB (2017) Gas hydrates formation phase boundary behaviour of synthetic natural gas system of the keta basin of Ghana. Open Pet Eng Journal 10:64–72
Kelland MW, Ke W (2016) Kinetic hydrate inhibitor studies for gas hydrate systems: a review of experimental equipment and test methods. Energy Fuels 30:10015–10028
Feng Y, Hu S, Liu X, Lo G, Zhu G (2014) Prevention and disposal technologies of gas hydartes in high sulfur gas reservoirs containing CO2. J Nat Gas Sci Eng 19:344–349
Ding T, Liu Y (2014) Simulations and analysis of hydrate formation during CO2 injection into cold saline aquifers. Energy Proced 63:3030–3040
Lim LV, Lloren AV, Lamorena RB (2014) The effect of urea in the nucleation process of CO2 clatharate hydrates. J Mol Liq 194:245–250
Chun LK, Jaafar A (2013) Ionic liquids as low dosage hydrate inhibitor for flow assurance in pipeline. Asian J Sci Res 6(2):374–380
Sa JH, Kwak JH, Lee BR, Park DH, Han K, Lee KH (2013) Hydrophobic amino acids as a new class of kinetic inhibitors for gas formation. Sci Rep 3:2428
Carey FA, Giuliano RM (2010) Organic chemistry, 18th edn. McGraw-Hill, New York
Naeiji P, Arjomandi A, Varaminian F (2014) Amino acids as kinetic inhibitors for tetrahydrofuran hydrate formation: experimental study and kinetic modeling. J Nat Gas Sci Eng 21:64–70
Bavoh CB, Nashed O, Khan MS, Partoon B, Lal B, Sharif AM (2018) The impact of amino acids on methane hydrate phase boundary and formation kinetics. J Chem Thermodyn 117:48–53
Longinos SN, Parlaktuna M (2021) Are the amino acids inhibitors or promoters on methane (95%)—propane (5%) hydrate formation? Reac Kinet Mech Cat 132(2):795–809. https://doi.org/10.1007/s11144-021-01959-0
Roosta H, Dashti A, Mazloumi SH, Varaminian F (2018) The dual effect of amino acids on the nucleation and growth rate of gas hydrate in ethane+ water, methane+ propane+ water and methane+ THF+ water systems. Fuel 212:151–161
Englezos P, Kalogerakis N, Dholabhai PD, Bishnoi PR (1987) Kinetics of formation of methane and ethane gas hydrates. Chem Eng Sci 42(11):2647–2658
Linga P, Daraboina N, Ripmeester JA, Englezos P (2012) Enhanced rate of gas hydrate formation in a fixed bed column filled with sand compared to a stirred vessel. Chem Eng Sci 68(1):617–623
Zhao J, Liu Y, Yang L, Zhang L, Song Y (2021) Organics-coated nanoclays further promote hydrate formation kinetics. J Phys Chem Lett 12(13):3464–3467
Liang H, Yang L, Song Y, Zhao J (2020) New approach for determining the reaction rate constant of hydrate formation via X-ray computed tomography. J Phys Chem C 125(1):42–48
Sun T, Davies PL, Walker VK (2015) Article structural basis for the inhibition of gas hydrates by α-helical antifreeze proteins. Biophys J 109:1698–1705
Bavoh BC, Partoon B, Lal B, Gonfa G, Khor FS, Sharif AM (2017) Inhibition effect of amino acids on carbon dioxide hydrate. J Chem Eng Sci 171:331–339
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:230–235
Roosta H, Dashti A, Mazloumi H, Varaminian F (2016) Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide water system: experimental ans modelling investigations. J Mol Liq 215:656–663
Sa JH, Lee BR, Park DH, Han K, Chun HD, Lee KH (2011) Amino acids as natural inhibitors for hydrate formation. Environ Sci Technol 45(13):5885–5891
Longinos SN, Parlaktuna M (2021) The effect of experimental conditions on methane hydrate formation by the use of single and dual impellers. Reac Kinet Mech Cat 132(2):771–794. https://doi.org/10.1007/s11144-021-01937-6
Longinos SN, Parlaktuna M (2021) Kinetic analysis of methane—propane hydrate formation by the use of different impellers. ASC Omega 6:1636–1646. https://doi.org/10.1021/acsomega.0c05615
Longinos SN, Parlaktuna M (2020) The effect of experimental conditions on methane (95%)–propane (5%) hydrate formation. Energies 13(24):6710. https://doi.org/10.3390/en13246710
Douieb S, Fradette L, Bertrand F, Haunt B (2015) Impact of the fluid flow conditions on the formation rate of carbon dioxide hydrates in as semi-batch stirred tank reactor. AIChE J 61(12):4387–4401
Longinos SN, Parlaktuna M (2021) Kinetic analysis of dual impellers on methane hydrate formation. Int J Chem React Eng 19(2):155–165. https://doi.org/10.1515/ijcre-2020-0231
Longinos SN, Parlaktuna M (2021) Kinetic analysis of CO2 hydrate formation by the use of different impellers. Reac Kinet Mech Cat 133(1):85–100. https://doi.org/10.1007/s11144-021-01968-z
Longinos SN, Parlaktuna M (2021) Examination of behavior of lysine on methane (95%)—propane (5%) hydrate formation by the use of different impellers. J Pet Explor Prod Technol. https://doi.org/10.1007/s13202-021-01146-w
Longinos SN, Parlaktuna M (2021) Examination of methane hydrate formation by the use of dual impeller combinations. React Kinet Mech Catal. https://doi.org/10.1007/s11144-021-02017-5
Longinos SN, Parlaktuna M (2021) Kinetic analysis of arginine, glycine and valine on methane (95%) – propane (5%) hydrate formation. React Kinet Mech Catal. https://doi.org/10.1007/s11144-021-02018-4
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Longinos, S.N., Parlaktuna, M. Kinetic study of the effect of amino acids on methane (95%)—propane (5%) hydrate formation. Reac Kinet Mech Cat 133, 753–763 (2021). https://doi.org/10.1007/s11144-021-02023-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02023-7