Abstract
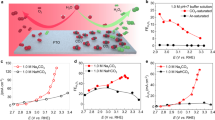
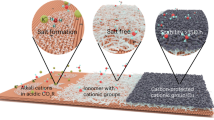
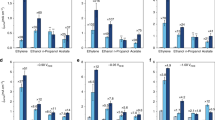
The electrochemical reduction of CO2 to value-added products is a promising approach for using CO2. However, the products are limited to reduced forms, such as CO, HCOOH and C2H4. Decreasing the anodic overpotential and designing membrane-separated systems are important determinants of the overall efficiency of the process. In this study we explored the use of redox-neutral reactions in electrochemical CO2 reduction to expand the product scope and achieve higher efficiency. We combined the CO2 reduction reaction with two redox cycles in an undivided cell so that the input electrons are carried through the electrolyte rather than settling in CO2. As a result, dimethyl carbonate—a useful fuel additive—has been synthesized directly from CO2 in methanol solvent with a Faradaic efficiency of 60% at room temperature. Our study shows that the formation of methoxide intermediates and the cyclic regeneration of the uniformly dispersed palladium catalyst by in situ-generated oxidants are important for dimethyl carbonate synthesis at room temperature. Furthermore, we successfully synthesized diethyl carbonate from CO2 and ethanol, demonstrating the generality and expandability of our system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and Supplementary Information files.
References
Jeong, H. Y. et al. Achieving highly efficient CO2 to CO electroreduction exceeding 300 mA cm–2 with single-atom nickel electrocatalysts. J. Mater. Chem. A 7, 10651–10661 (2019).
Philips, M. F., Gruter, G. J. M., Koper, M. T. M. & Schouten, K. J. P. Optimizing the electrochemical reduction of CO2 to formate: a state-of-the-art analysis. ACS Sustain. Chem. Eng. 8, 15430–15444 (2020).
Balamurugan, M. et al. Electrocatalytic reduction of CO2 to ethylene by molecular Cu-complex immobilized on graphitized mesoporous carbon. Small 16, 2000955 (2020).
Weng, L. C., Bell, A. T. & Weber, A. Z. Towards membrane-electrode assembly systems for CO2 reduction: a modeling study. Energy Environ. Sci. 12, 1950–1968 (2019).
Garg, S. et al. Advances and challenges in electrochemical CO2 reduction processes: an engineering and design perspective looking beyond new catalyst materials. J. Mater. Chem. A 8, 1511–1544 (2020).
Liu, A. et al. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts. J. Mater. Chem. A 8, 3541–3562 (2020).
Mo, Y. et al. Microfluidic electrochemistry for single-electron transfer redox-neutral reactions. Science 368, 1352–1357 (2020).
Liang, S. et al. Electrochemically catalyzed amino-oxygenation of styrenes: n-Bu4NI induced C–N followed by a C–O bond formation cascade for the synthesis of indolines. Green. Chem. 18, 2222–2230 (2016).
Jud, W., Kappe, C. O. & Cantillo, D. Catalyst-free oxytrifluoromethylation of alkenes through paired electrolysis in organic-aqueous media. Chem. Eur. J. 24, 17234–17238 (2018).
Ma, Y. et al. Direct arylation of α-amino C(sp3)-H bonds by convergent paired electrolysis. Angew. Chem. Int. Ed. 58, 16548–16552 (2019).
Kawamata, Y. et al. Electrochemically driven, Ni-catalyzed aryl amination: scope, mechanism, and applications. J. Am. Chem. Soc. 141, 6392–6402 (2019).
Esan, A. O., Adeyemi, A. D. & Ganesan, S. A review on the recent application of dimethyl carbonate in sustainable biodiesel production. J. Clean. Prod. 257, 120561 (2020).
Abdalla, A. O. G. & Liu, D. Dimethyl carbonate as a promising oxygenated fuel for combustion: a review. Energies 11, 1552 (2018).
Xie, J. & Lu, Y. C. A retrospective on lithium-ion batteries. Nat. Commun. 11, 2499 (2020).
Selva, M., Perosa, A., Rodríguez-Padrón, D. & Luque, R. Applications of dimethyl carbonate for the chemical upgrading of biosourced platform chemicals. ACS Sustain. Chem. Eng. 7, 6471–6479 (2019).
Dimethyl Carbonate Market by Application, End-Use Industry, Grade, Region — Global Forecast to 2025 (Markets and Markets, 2021).
Bansode, A. & Urakawa, A. Continuous DMC synthesis from CO2 and methanol over a CeO2 catalyst in a fixed bed reactor in the presence of a dehydrating agent. ACS Catal. 4, 3877–3880 (2014).
Zhang, M. et al. Continuous dimethyl carbonate synthesis from CO2 and methanol using Cu-Ni@VSiO as catalyst synthesized by a novel sulfuration method. Catalysts 8, 142 (2018).
Verma, S., Baig, R. B. N., Nadagouda, M. N. & Varma, R. S. Fixation of carbon dioxide into dimethyl carbonate over titanium-based zeolitic thiophene-benzimidazolate framework. Sci. Rep. 7, 655 (2017).
Santos, B. A. V., Silva, V. M. T. M., Loureiro, J. M. & Rodrigues, A. E. Review for the direct synthesis of dimethyl carbonate. ChemBioEng Rev. 1, 214–229 (2014).
Wu, T. W. & Chien, I. L. CO2 utilization feasibility study: dimethyl carbonate direct synthesis process with dehydration reactive distillation. Ind. Eng. Chem. Res. 59, 1234–1248 (2020).
Kumar, P., Srivastava, V. C. & Štangar, U. L. Recent progress in dimethyl carbonate synthesis using different feedstock and techniques in the presence of heterogeneous catalysts. Catal. Rev. 1–59 (2019).
Li, Y. et al. An efficient route for electrooxidation of methanol to dimethoxymethane using ionic liquid as electrolyte. J. Electrochem. Soc. 164, H5074–H5077 (2017).
Zhao, S., Jin, R. & Jin, R. Opportunities and challenges in CO2 reduction by gold- and silver-based electrocatalysts: from bulk metals to nanoparticles and atomically precise nanoclusters. ACS Energy Lett. 3, 452–462 (2018).
Liu, Q., Takemura, F. & Yabe, A. Solubility and diffusivity of carbon monoxide in liquid methanol. J. Chem. Eng. Data 41, 589–592 (1996).
Wu, X. F., Neumann, H. & Beller, M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem 6, 229–241 (2013).
Xinbin, M. A. et al. Effect of Cu catalyst preparation on the oxidative carbonylation of methanol to dimethyl carbonate. React. Kinet. Catal. Lett. 76, 179–187 (2002).
Wang, Z. Q., Sun, J., Xu, Z. N. & Guo, G. C. CO direct esterification to dimethyl oxalate and dimethyl carbonate: the key functional motifs for catalytic selectivity. Nanoscale 12, 20131–20140 (2020).
Han, B. et al. CO oxidative coupling to dimethyl oxalate over Pd–Me (Me = Cu, Al) catalysts: a combined DFT and kinetic study. Phys. Chem. Chem. Phys. 20, 7317–7332 (2018).
Ling, L. et al. The catalytic CO oxidative coupling to dimethyl oxalate on Pd clusters anchored on defected graphene: a theoretical study. Mol. Catal. 453, 100–112 (2018).
Lv, D. M. et al. (Pd–CuCl2)/γ-Al2O3: a high-performance catalyst for carbonylation of methyl nitrite to dimethyl carbonate. Catal. Sci. Technol. 5, 3333–3339 (2015).
Vuong, K. Q. et al. Dialkyl carbonate synthesis via in situ generated carbonyl dibromide on porous glass. ACS Sustain. Chem. Eng. 5, 7492–7495 (2017).
Brown, H. C. & Lane, C. F. Organoboranes for synthesis. 10.1 the base-induced reaction of bromine with organoboranes. A convenient procedure for the conversion of alkenes into alkyl bromides via hydroboration. Tetrahedron 44, 2763–2772 (1988).
Zhuang, T. T. et al. Dopant-tuned stabilization of intermediates promotes electrosynthesis of valuable C3 products. Nat. Commun. 10, 4807 (2019).
Otsuka, K., Yagi, T. & Yamanaka, I. Dimethyl carbonate synthesis by electrolytic carbonylation of methanol in the gas phase. Electrochim. Acta 39, 2109–2115 (1994).
Otsuka, K., Yagi, T. & Yamanaka, I. Electrolytic carbonylation of methanol over the CuCl2 anode in the gas phase. J. Electrochem. Soc. 142, 130–135 (1995).
Funakawa, A., Yamanaka, I. & Otsuka, K. Active control of methanol carbonylation selectivity over Au/carbon anode by electrochemical potential. J. Phys. Chem. B 109, 9140–9147 (2005).
Yamanaka, I., Funakawa, A. & Otsuka, K. Electrocatalytic synthesis of DMC over the Pd/VGCF membrane anode by gas–liquid–solid phase-boundary electrolysis. J. Catal. 221, 110–118 (2004).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Kongpanna, P., Pavarajarn, V., Gani, R. & Assabumrungrat, S. Techno-economic evaluation of different CO2-based processes for dimethyl carbonate production. Chem. Eng. Res. Des. 93, 496–510 (2015).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Huang, S., Yan, B., Wang, S. & Ma, X. Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev. 44, 3079–3116 (2015).
Shukla, K. & Srivastava, V. C. Diethyl carbonate: critical review of synthesis routes, catalysts used and engineering aspects. RSC Adv. 6, 32624–32645 (2016).
Li, D., Fang, W., Xing, Y., Guo, Y. & Lin, R. Effects of dimethyl or diethyl carbonate as an additive on volatility and flash point of an aviation fuel. J. Hazard. Mater. 161, 1193–1201 (2009).
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT; NRF-2017R1A2B3012003 and NRF-2018M3C1B7021994), the KIST Institutional Program (NRF-2020M3H4A1A02084594), the Creative Materials Discovery Program through the NRF, funded by the Ministry of Science and ICT (NRF-2017M3D1A1039377) and the Korea Environment Industry & Technology Institute (KEITI) through the Ecological Imitation-based Environmental Pollution Management Technology Development Project, funded by the Korea Ministry of Environment (MOE; 2021002800009). K.T.N. appreciates support from the Institute of Engineering Research, the Research Institute of Advanced Materials (RIAM) and the Soft Foundry at Seoul National University.
Author information
Authors and Affiliations
Contributions
K.M.L., J.H.J. and K.T.N. designed the research and wrote the manuscript. K.M.L., J.H.J. and M.B. conducted the electrochemical experiments and optimized the DMC synthesis conditions. K.M.L., J.H.J. and J.E.K. performed the mechanistic investigation and analysed the data. K.M.L., J.H.J. and Y.I.J. investigated the Br2 loss mechanism. All authors discussed the experiments and contributed to the writing of this manuscript. K.T.N. guided all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Additional information
Peer review information Nature Energy thanks Markus Kärkäs, Marc Robert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary Figs. 1–15 and Table1.
Rights and permissions
About this article
Cite this article
Lee, K.M., Jang, J.H., Balamurugan, M. et al. Redox-neutral electrochemical conversion of CO2 to dimethyl carbonate. Nat Energy 6, 733–741 (2021). https://doi.org/10.1038/s41560-021-00862-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-021-00862-1
This article is cited by
-
Catalytic carbon–carbon bond cleavage in lignin via manganese–zirconium-mediated autoxidation
Nature Communications (2024)
-
Graphene-anchored sodium single atoms: A highly active and stable catalyst for transesterification reaction
Nano Research (2024)
-
Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction
Nature Communications (2023)
-
Magnesium single-atom catalysts with superbasicity
Science China Chemistry (2023)
-
Recent advances in cycloaddition of CO2 with epoxides: halogen-free catalysis and mechanistic insights
Frontiers of Chemical Science and Engineering (2023)