Abstract
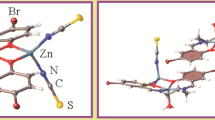
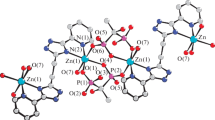
The reaction of zinc chloride with 2-benzylideneamino-5-methylphenol (LH), a bidentate Schiff base, in 1 : 1 molar ratio in the presence of triethylamine gives the ionic complex [Et3NH]+[LZnCl2]– (I). The reaction between the zinc salt (chloride or acetate) and LH in 1 : 2 ratio results in complete displacement of the zinc salt anions to give bis-o-iminophenolate ZnL2 (II), which exists in the crystalline state as the (ZnL2)2 dimer formed via μ-bridging oxygen atoms of two out of the four o-iminophenolate ligands. The molecular structure of ligand LH and complexes I and (II·CDCl3)2 was established by X-ray diffraction (CIF files CCDC nos. 2052647 (LH), 2052645 (I), 2052646 (II·CDCl3)2).


Similar content being viewed by others
REFERENCES
Vigato, P.A. and Tamburini, S., Coord. Chem. Rev., 2004, vol. 248, nos. 17–20, p. 1717.
Liu, X. and Hamon, J.-R., Coord. Chem. Rev., 2019, vol. 389, p. 94.
Miroslaw, B., Int. J. Mol. Sci., 2020, vol. 21, no. 10, p. 3493.
Clarke, R.M. and Storr, T., Dalton Trans., 2014, vol. 43, no. 25, p. 9380.
Liu, X., Manzur, C., Novoa, N., et al., Coord. Chem. Rev., 2018, vol. 357, p. 144.
Zoubi, W.A., Al-Hamdani, A.A.S., and Kaseem, M., Appl. Organomet. Chem., 2016, vol. 30, no. 10, p. 810.
Gupta, K.C. and Sutar, A.K., Coord. Chem. Rev., 2008, vol. 252, nos. 12–14, p. 1420.
Hameed, A., Rashida, M., Uroos, M., et al., Exp. Opin. Ther. Pat., 2017, vol. 27, no. 1, p. 63.
Hossain, M.S., Roy, P.K., Zakaria, C.M., et al., Int. J. Chem. Stud., 2018, vol. 6, no. 1, p. 19.
Kumar, M., Abbas, Z., Tuli, H.S., and Rani, A., Curr. Pharmacol. Rep., 2020, vol. 6, p. 167.
Kaczmarek, M.T., Zabiszak, M., Nowak, M., and Jastrzab, R., Coord. Chem. Rev., 2018, vol. 370, p. 42.
More, M.S., Joshi, P.G., Mishra, Y.K., and Khanna, P.K., Mater. Today Chem., 2019, vol. 14, p. 100195.
Malik, M.A., Dar, O.A., Gull, P., et al., Med. Chem. Commun., 2018, vol. 9, no. 3, p. 409.
Berhanu, A.L., Gaurav, I., Mohiuddin, A.K., et al., Trends Anal. Chem., 2019, vol. 116, p. 74.
Zhang, J., Xu, L., and Wong, W.-Y., Coord. Chem. Rev., 2018, vol. 355, p. 180.
Oiye, É.N., Ribeiro, M.F.M., Katayama, J.M.T., et al., CRC Crit. Rev. Anal. Chem., 2019, vol. 49, no. 6, p. 488.
Udhayakumari, D. and Inbaraj, V., J. Fluoresc., 2020, vol. 30, p. 1203.
Garnovsky, A.D., Antsyshkina, A.S., Sadikov, G.G., et al., Russ. J. Inorg. Chem., 1995, vol. 40, p. 64.
Kim, Y.-I., Yun, S.-J., Hwang, I.-H., et al., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, vol. 68, p. m504.
Gordon, A. and Ford, R., The Chemist’s Companion: A Handbook of Practical Data, Techniques, and References, New York: Wiley, 1972.
Bruker. APEX3, Madison: Bruker AXS Inc., 2015.
Rigaku Oxford Diffraction. CrysAlisPro. Version 1.171.38.46, Wroclaw: Rigaku Corporation, 2015.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, no. 1, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, no. 1, p. 3.
Fedushkin, I.L., Tishkina, A.N., Fukin, G.K., et al., Eur. J. Inorg. Chem., 2008, no. 3, p. 483.
Fedushkin, I.L., Skatova, A.A., Ketkov, S.Y., et al., Angew. Chem., Int. Ed. Engl., 2007, vol. 46, p. 4302.
Batsanov, S.S. Russ. J. Inorg. Chem., 1991, vol. 36, p. 1694.
Briel, O., Fehn, A., Polborn, K., and Beck, W., Polyhedron, 1999, vol. 18, nos. 1–2, p. 225.
Novoa, N., Roisnel, T., and Hamon, P., Dalton Trans., 2015, vol. 44, no. 41, p. 18019.
Perez, S., Lopez, C., Caubet, A., et al., J. Organomet. Chem., 2007, vol. 692, p. 2402.
Pérez, S., López, C., Caubet, A., et al., Eur. J. Inorg. Chem., 2008, vol. 2008, p. 1599.
Baryshnikova, S.V., Poddel’sky, A.I., Cherkasov, A.V., and Smolyaninov, I.V., Inorg. Chim. Acta, 2019, vol. 495, p. 118963.
Baryshnikova, S.V., Bellan, E.V., Poddel’sky, A.I., et al., Inorg. Chem. Commun., 2016, vol. 69, p. 94.
Baryshnikova, S.V., Bellan, E.V., Poddel’sky, A.I., et al., Eur. J. Inorg. Chem., 2016, vol. 2016, no. 33, p. 5230.
Poddel’sky, A.I., Arsenyev, M.V., Astaf’eva, T.V., et al., J. Organomet. Chem., 2017, vol. 835, p. 17.
Poddel’sky, A.I. Astaf’eva, T.V., et al., J. Organomet. Chem., 2018, vol. 873, p. 57.
Baryshnikova, S.V., Poddel’sky, A.I., Bellan, E.V., et al., Inorg. Chem., 2020, vol. 59, no. 10, p. 6774.
Elmali, A., Elerman, Y., Zeyrek, C.T., and Svoboda, I., Z. Naturforsch. B: J. Chem. Sci., 2003, vol. 58, no. 5, p. 433.
Wong, J.L., Sanchez, R.H., Logan, J.G., et al., Chem. Sci., 2013, vol. 4, no. 4, p. 1906.
Klementyeva, S.V., Smolentsev, A.I., Abramov, P.A., and Konchenko, S.N., Inorg. Chem. Commun., 2017, vol. 86, p. 154.
Klementyeva, S.V., Lukoyanov, A.N., Afonin, M.Yu., et al., Dalton Trans., 2019, vol. 48, p. 3338.
Piskunov, A.V., Maleeva, A.V., Meshcheryakova, I.N., and Fukin, G.K., Russ. J. Coord. Chem., 2013, vol. 39, p. 245. https://doi.org/10.1134/S107032841303007X
Meshcheryakova, I.N., Shavyrin, A.S., Cherkasov, A.V., and Piskunov, A.V., Izv. Akad. Nauk, Ser. Khim., 2019, no. 7, p. 1414.
ACKNOWLEDGMENTS
The studies were performed using research equipment of the Center for Collective Use “Analytical Center of the Razuvaev Institute of Organometallic Chemistry, Russian Academy of Sciences” at the Razuvaev Institute of Organometallic Chemistry, Russian Academy of Sciences.
Funding
The studies of zinc salt reactions with o-iminophenol were supported by the Russian Foundation for Basic Research (project no. 19-03-00208a); spectroscopic studies were carried out within the framework of state assignment for the Razuvaev Institute of Organometallic Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Protasenko, N.A., Baryshnikova, S.V., Astaf’eva, T.V. et al. Mono- and Binuclear Zinc Complexes with a Bidentate Phenol-Containing 2-Benzylideneamino-5-Methylphenol Schiff Base. Russ J Coord Chem 47, 417–423 (2021). https://doi.org/10.1134/S1070328421060038
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328421060038