Phylogenetic Diversity of Urban Floras in the Central Urals
- 1Department of Biodiversity and Bioecology, Institute of Natural Sciences and Mathematics, Ural Federal University, Yekaterinburg, Russia
- 2Department of Ecology, Institute of Biology and Biomedicine, Lobachevsky State University of Nizhni Novgorod, Nizhni Novgorod, Russia
- 3Department of Biology and Fundamental Medicine, Institute of Natural Sciences and Mathematics, Ural Federal University, Yekaterinburg, Russia
- 4Laboratory of Experimental Ecology and Acclimatization of Plants, Institute Botanic Garden of Ural Branch of the Russian Academy of Sciences, Yekaterinburg, Russia
- 5Department of Biological Sciences, University of Toronto Scarborough, Toronto, ON, Canada
Modern cities harbor a high diversity of plants, and urban floras are significantly different from non-urban floras especially when considering the proportion of alien species found in cities. However, it is not clear whether urban areas disproportionately select for species from relatively few evolutionary lineages or provide opportunities for species across the full spectrum of plant lineages. Here, we examined the taxonomic and phylogenetic diversity of the floras in four cities (Yekaterinburg, Kamensk-Uralsky, Krasnoufimsk, and Turinsk) in the understudied region of Central Urals (Russian Federation). We classified native species into indigenous and apophytic species, namely, those that are sensitive to anthropogenic disturbance and those that have expanded their range with human activity, respectively. Alien species were classified into archaeophytes and neophytes according to when they were introduced (i.e., before or after than 1800). Phylogenetic diversity was quantified using Faith’s index to reflect total evolutionary history in urban areas and mean phylogenetic distance (MPD) to reflect species dissimilarity. Phylogenetic diversity of native species was higher than that for alien species, and the standardized effect size (SES) of MPD for natives was positive, reflecting their general dissimilarity from one another, while it was very negative for aliens, showing that they were phylogenetically clustered. However, among natives, apophytes were significantly clustered, while indigenous species were overdispersed. For the aliens, MPD was higher for archaeophytes compared to neophytes, though both groups were significantly clustered. These results show that urbanization leads to a non-random selection of plants. Apophytes and alien plants were composed of closely related species, reflecting similar ecological traits and are likely to be pre-adapted to the environmentally altered and highly disturbed urban environment.
Introduction
Anthropogenic activity has weakened natural biogeographic barriers that limit the distributions of plants (Kueffer, 2017; Potgieter and Cadotte, 2020). These activities have intensified species migration and have contributed to the establishment of alien plant assemblages in the floras of many different geographical areas (Lonsdale, 1999; Aronson et al., 2016). The highest numbers of alien plants are concentrated in urban floras where purposeful introductions and environmental changes are most apparent (Aronson et al., 2014; Cadotte et al., 2017; Potgieter and Cadotte, 2020). Globally, it has been shown that cities generally harbor a high diversity of alien plant species; frequently between 25 and 50% of urban floras are classified as alien (Ricotta et al., 2009; Cadotte, 2020). The presence of alien plants influences the overall composition and structure of urban floras (Ricotta et al., 2009, 2012; Cadotte, 2020). The net effect of urban environmental conditions, human preferences, and intensified species immigration are hypothesized to be major drivers of biotic homogenization in cities (McKinney and Lockwood, 1999; Olden et al., 2004; McKinney, 2006; La Sorte et al., 2007).
However, taxonomic diversity does not directly reveal ecological patterns. It cannot reveal whether harsh urban environments select for similar species or whether species differences support coexistence (Cavender-Bares et al., 2009; Cadotte and Tucker, 2017). Biotic homogenization should be driven by two distinct mechanisms. First, species benefiting from urbanization should increase the similarity among urban floras. Second, if urbanization selects for specific traits and niches (e.g., Zhu et al., 2019), then relatively few, non-random groups should be driving this homogenization.
To account for such non-random species composition, different facets of biodiversity have been considered, including phylogenetic diversity, which takes into account the phylogenetic relationships between species based on the evolutionary relationships connecting all species together (Cadotte and Davies, 2016; Bitomský et al., 2020).
The use of phylogenies in ecology has expanded greatly over the past 20 years, and the reasons to use this information are twofold. First is that phylogenies provide direct information about how evolutionary history and specifically speciation events shape local diversity (Gerhold et al., 2015), thus revealing the ways in which human activities reshape the influence of this evolutionary history. Second is that it is often inferred that phylogenies are a good surrogate for functional, ecological, or niche diversity, especially when we conceive of functional diversity as including large numbers of traits in multivariate contexts (Tucker et al., 2018). While the relationship between phylogenetic and functional diversity can be complex and influenced by methodological decisions and ecological processes (Cadotte et al., 2019), this surrogacy has been shown to be the case in urban areas (Lososová et al., 2016), and so species expanding their ranges with urbanization likely result in reduced functional space relative to the native habitats that existed prior to human settlement and urban expansion. Furthermore, given the importance of ecosystem function and the delivery of ecosystem services in urban areas, reduced phylogenetic and functional diversity might result in lower functioning (Flynn et al., 2011; Cadotte, 2013, 2017).
Phylogenetic analysis can reveal non-random patterns based on speciation and biogeography or from ecological processes that select for certain species. Evolutionarily, we might expect that the native flora is the product of relatively few successful clades and would therefore be relatively closely related, while alien species are a selection across many clades and regions and therefore represent higher phylogenetic diversity. Conversely, urban stressors and disturbances might select for species with the requisite adaptations allowing them to persist, resulting in both groups being phylogenetically underdispersed or clustered. A few studies have shown that alien species are phylogenetically less diverse than natives in cities (e.g., Čeplová et al., 2015; Knapp et al., 2017; Zhu et al., 2019), but there are surprisingly few such analyses.
However, native and alien floras themselves are heterogeneous assemblages and subdividing them in meaningful ways can further reveal how urbanization influences plant composition. The native flora can be separated into species that appear to be sensitive to human activity and mostly persist in natural ecosystems (i.e., what we refer to as indigenous) and into those that appear to have expanded their range and abundance by flourishing in human-dominated landscapes (i.e., apophytes) (Sukopp, 2006). We should expect that apophytes are more closely related than other species because they possess traits and strategies that are better suited to urban environments. The alien flora can be divided into species that assimilated into the regional flora long ago (i.e., archaeophytes) and those that were introduced more recently (i.e., neophytes) (Pysek et al., 2004). In an analysis of urban floras in Europe and the United States, Ricotta et al. (2009, 2012) showed that the phylogenetic diversity of alien species was significantly lower than that of native species, and archaeophytes were characterized by lower phylogenetic diversity compared to neophytes. A potential reason is that neophytes are more likely to be planted and supported by gardening in cities more recently (after 1800) because of their uniqueness, while archaeophytes are those species with pre-adaptations that allow them to persist or thrive after accidental introduction through early trade and agricultural expansion.
Despite the size of Russia, the diversity of cities, and its long history, analyses of urban biodiversity in Russian cities have not appeared in many international journals. Comparative analyses of the taxonomic structure of native and alien plants of urban floras in Russia have been performed and primarily published in Russian journals and monographs (Grigoryevskaya, 2000; Terekhina, 2000; Antipina, 2002; Panasenko, 2003; Sutkin, 2006; Tretyakova and Shurova, 2013; Senator et al., 2015; Antipova and Antipova, 2016; Golovanov and Abramova, 2017), and the patterns uncovered in this region can help inform our understanding of urbanization impacts on biodiversity globally.
Across Russia, species richness of floras for cities with more than 1,000,000 people is about 1,200 species on average, about 800 for cities with 100,000–1,000,000, and about 500 species for towns with fewer than 100,000 people; the proportion of alien plants in Russian cities reaches 30–50% (Veselkin et al., 2017; Tretyakova et al., 2018). Urban floras are characterized by high taxonomic diversity, often exceeding diversity compared to the non-urban flora in the same regions (Tretyakova, 2016). There are obvious differences in the taxonomic composition of urban floras in Russian cities compared to non-urban floras of the same region. Species diversity of spore plants, gymnosperms, and monocotyledons is lower in urban floras, as well as the proportion of representatives of certain families of dicots that are typical in the boreal flora (Tolmachev, 1974; Khokhryakov, 2000). At the same time, families that tend to be affiliated with disturbed habitats are over-represented in urban floras (Terekhina, 2000; Antipina, 2002; Beresutsky and Panin, 2007; Antipova and Antipova, 2016; Tretyakova, 2016; Golovanov and Abramova, 2017).
In Russia, the phylogenetic diversity of urban flora has not yet been considered and so the goal of the present study was to characterize the phylogenetic diversity of native and alien plants in the urban floras of Sverdlovsk region in the Central Urals. This region was selected due to the high level of urbanization (proportion of urban population is about 88%) and its location on the transition zone between taiga and forest-steppe biomes. We expect (1) higher similarity of apophytes and archaeophytes among cities because these groups are composed of species adapted to disturbed habitats. We hypothesize that (2) alien species will exhibit lower phylogenetic diversity compared to native species since both alien and native species are non-random sets of species: aliens are able to excel under human preference and urban environmental filters and are thus likely drawn from fewer clades while natives are adapted to a wider range of conditions and are thus likely to be drawn from more disparate clades. We further hypothesize (3) apophytes to show similar patterns to aliens and exhibit low phylogenetic diversity compared to indigenous species, again because they are non-random subsets that can thrive under human influences. Finally, we expect (4) archaeophytes to have lower phylogenetic diversity than neophytes, since the latter are likely conscientiously drawn from more diverse species pools from around the world.
Materials and Methods
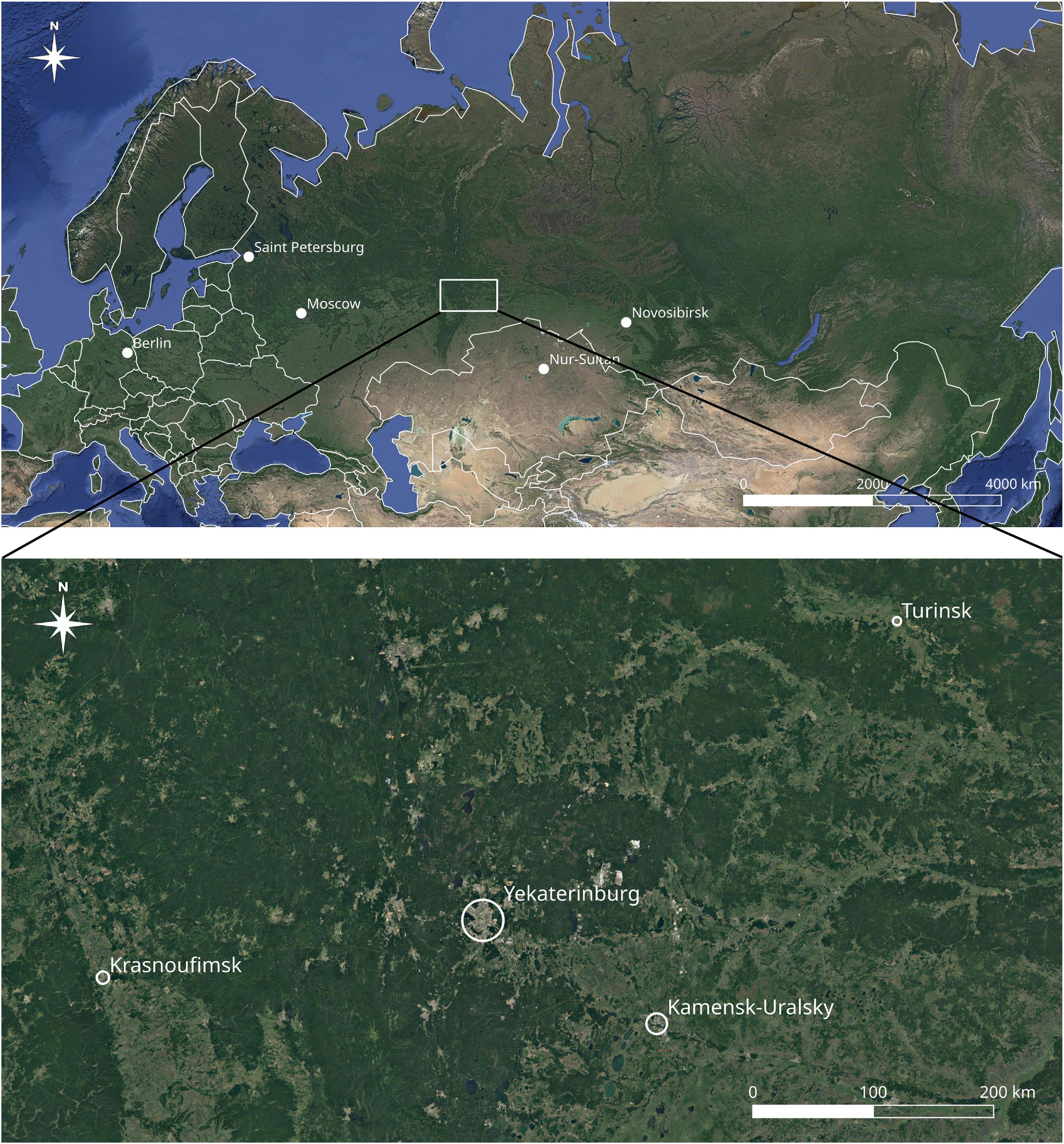
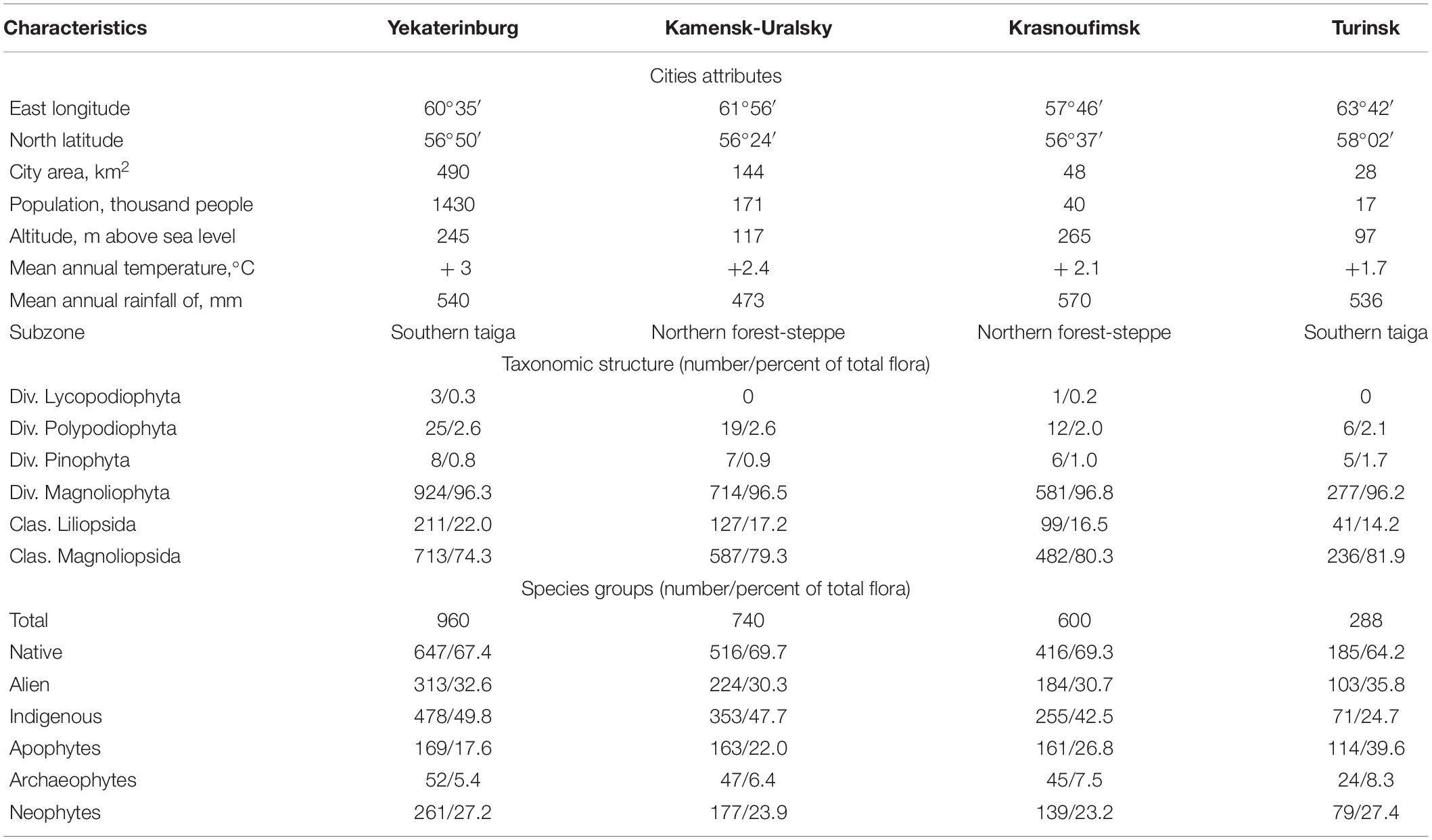
Four cities were selected for our analysis of urban floraphylogenetic diversity: Krasnoufimsk, Yekaterinburg, Kamensk-Uralsky, and Turinsk (Figure 1). The selected citiesdiffer from each other in terms of their geographic location, size, and level of economic development. Krasnoufimsk is located on the border of the western foothills of the Urals and the East European Plain, Yekaterinburg is on the border of the eastern foothills and the spine part of the Urals, Kamensk-Uralsky is on the border of the eastern foothills of the Urals and the West Siberian Plain, and Turinsk is on the West Siberian Plain. Together, they form a chain (from 56°37′N to 58°02′N and from 57°46′E to 63°42′E), which covers the Cis-Urals (geological features to the west of the Urals), mountain part of the Central Urals, Trans-Urals, and Western Siberia. In terms of population, the cities ranged from 17,000 to over 1.4 million residents (Table 1).
The floras of these cities were studied from 2000 to 2016. The urban floras include all vascular plant entities (divisions Lycopodiophyta, Polypodiophyta, Pinophyta, and Magnoliophyta) that occur spontaneously within the administrative boundaries of the cities. The urban flora for the Central Urals is based on field surveys performed by the authors. Every type of habitat, including both natural/semi-natural and humanmade (Figure 2), was thoroughly surveyed for the presence of species. Species lists are regularly updated based on new findings. These data were supplemented with the information from herbarium collections [Herbariums of the Institute of Plant and Animal Ecology of the Ural Branch of the Russian Academy of Sciences (SVER), Ural Federal University (UFU), and Kurgan State University] and published data on the flora of the Central Urals. The goal of these surveys and herbarium searches was to compile as complete a list as possible.
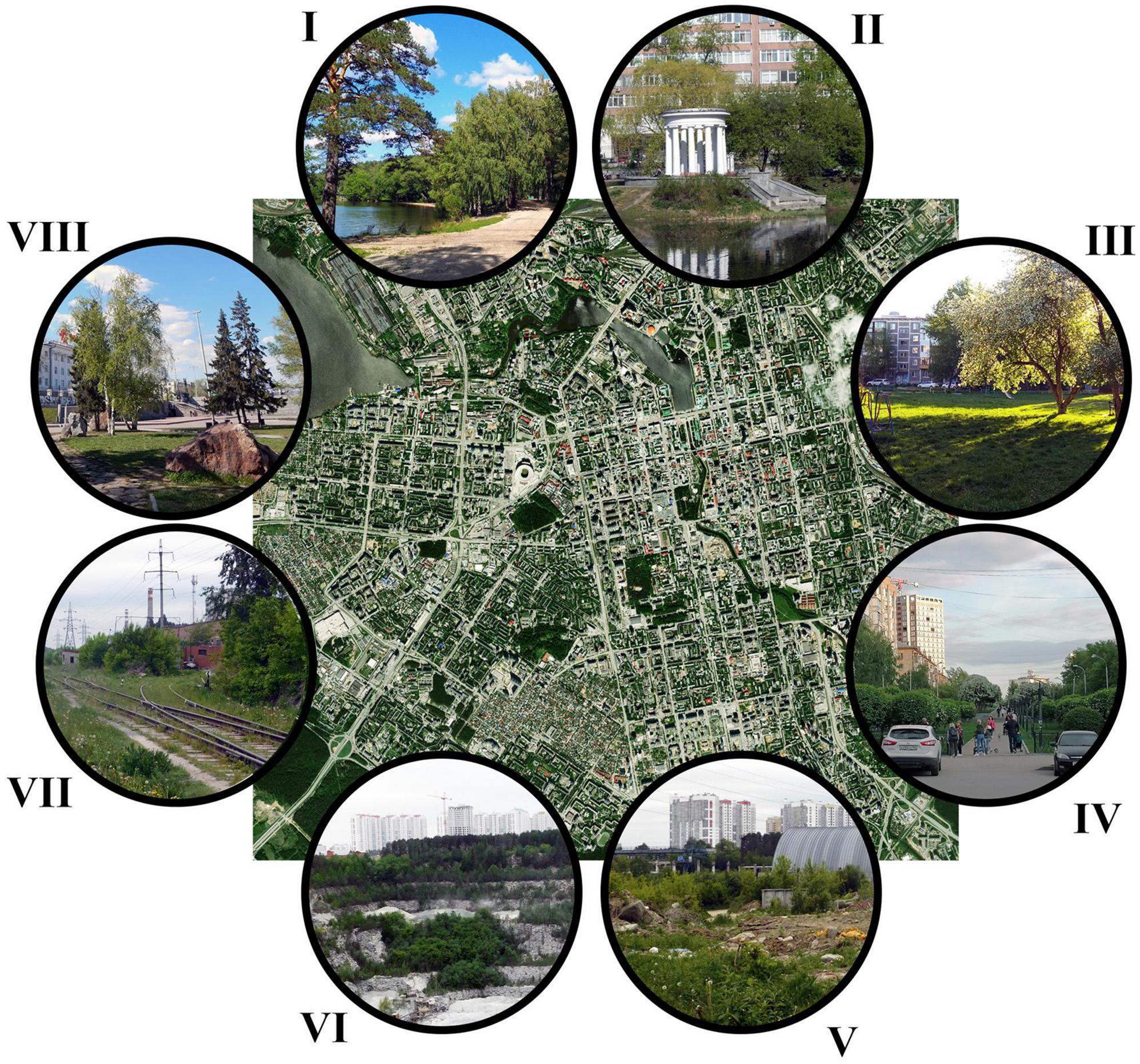
Figure 2. The variety of habitat types in the city of Yekaterinburg, including remnant natural areas (I, Shartashsky forest park), plantings in the city core (II, Kharitonovsky park; VIII, Historical square; IV, Mira street; III, community gardens), unmanaged vacant lots (V), crushed stone quarry (VI), and railway (VII). Base map: Yekaterinburg, Russia. Photo Credits: Andrey Gusev (I–II, IV–VIII) and Elena Zhuikova (III).
Native and alien species were distinguished in the urban flora using checklists of the flora of Sverdlovsk Region (Knyazev et al., 2016, 2017, 2018, 2019a, b, 2020) in which species status was evaluated based on the appearance in the studied area due to human activity. We categorized native species as either indigenous or apophytic based on whether they exhibited expanded ranges because of their association with disturbed habitat. The indigenous plants included species confined to natural communities and that generally avoid anthropogenic habitats while apophyte plants included anthropogenic habitat-associated species from the native flora. The assignment of plants to the selected groups was based on the species distribution analysis in natural and anthropogenic habitats in the urban habitats (see Tretyakova, 2014; Baranova et al., 2018).
We considered alien species as those species unintentionally introduced into the city territory, as well as decorative or purposefully introduced species, for which the presence of seed or vegetative propagules were observed, or plants found outside their cultivation sites (Tretyakova and Shurova, 2013; Baranova et al., 2018). Alien species were divided into two groups based on their residence time: archaeophytes and neophytes (Pysek et al., 2004). Archaeophytes are the alien species that appeared in the Central Urals before the arrival of the Russian population prior to 1800. Neophytes appeared in the Central Urals after this date. The checklist of the flora of Sverdlovsk region, parts I–VI (Knyazev et al., 2016, 2017, 2018, 2019a, b, 2020), served as a basic source for assignment to a groups of archaeophytes and neophytes. We included records from Flora Rossica by K. F. Ledebourg (von Ledebour et al., 1842–1853) that summarized information about the flora of the Urals, accumulated by the beginning of the 19th century.
Final lists of the studied urban floras, including grouping of species into indigenous plants, apophytes, neophytes, and archaeophytes, are available in the GBIF repository1.
When assessing the similarity of the species composition among urban floras, the Jaccard similarity coefficient was used, which was calculated as a/(a + b + c), where a is the number of species presented in both lists, and b and c are the numbers of unique species (Legendre and Legendre, 2012).
We used a combined dated phylogenetic tree published by Zanne et al. (2014), which includes 32,223 species of land plants to extract a phylogeny for our region. In the urban flora in the Central Urals, 1,035 plant species were recorded, and synonymy was aligned with The Plant List (The Plant List: URL2). Two hundred fifty-six of our observed plant species were absent in the phylogenetic tree, and so we added them as polytomies to the corresponding genera (239), and in the absence of genera, they were added to the respective families (17). These 256 species belong to 49 families, mostly Asteraceae (53 species), Rosaceae (20), Caryophyllaceae (18), and Poaceae (17).
To characterize the phylogenetic diversity in these urban floras, we calculated Faith’s PD, which sums the phylogenetic tree branch lengths that connect all species of a given urban flora or the corresponding grouping (e.g., native vs. alien). This index is a phylogenetic analog of species richness, and it is usually strongly correlated with it (Faith, 1992; Tucker et al., 2017). We also used a complementary phylogenetic diversity measure, the average pairwise phylogenetic distance between species [mean phylogenetic distance (MPD)], which is relatively insensitive to species richness and is well-suited to comparing phylogenetic and taxonomic diversity facets of different-sized species lists, as well as providing insights into the roles of different ecological mechanisms like environmental filtering and species interactions (Webb et al., 2002; Cadotte and Davies, 2016).
We compared the phylogenetic diversity in each of the species groups listed in Table 1 to null expectations generated from randomizations of species membership in each grouping for each city (Webb, 2000; Ricotta et al., 2009; Cadotte and Davies, 2016). The complete urban flora observed for each urban area was considered as a pool of species from which the plant classes were assembled. We estimated MPD for the random assemblages and repeated this 1,000 times. We then calculated a standardized effect size (SES) by subtracting the null model mean and dividing the difference by the null model standard deviation (Webb, 2000; Cadotte and Davies, 2016). Negative SES.MPD values correspond to phylogenetic clustering; in this case, MPD is lower compared to the null expectation, i.e., the group is composed of more related species. On the contrary, positive SES.MPD values indicate phylogenetic overdispersion. Null model analysis can also test significance of clustering or overdispersion effect. The corresponding p-value is calculated as a proportion of null distribution lesser than the empirical value of a metric. Clustering is considered to be significant when p < 0.025 and overdispersion is considered to be significant when p > 0.975 (two-tailed hypothesis; Swenson, 2014).
All calculations were performed in R version 4.0.2 (R Core Team, 2020). We used package picante (Kembel et al., 2010) to calculate all phylogenetic metrics including PD, MPD, and SES.MPD.
Results
The number of species recorded in these urban floras varied from 288 species in Turinsk to 960 species in Yekaterinburg. All major taxonomic groups of vascular plants are represented in these urban floras, but angiosperms (96.2–96.8%) and, specifically, dicotyledonous (74.3–81.9%) plants dominate these lists (see Table 1). Native plants constitute the majority of these urban floras. In the analyzed cities, with, on average, 67% of species being native, among them the indigenous class contained more species than apophytes. Alien plants account for about 30% of the urban species and were predominantly neophytes. Archaeophytes and neophytes account for 7% and 25% of all urban flora, respectively (see Table 1).
Flora similarity analysis revealed that species composition in the indigenous group was significantly more differentiated among cities than the apophytes (t = 5.24, p < 0.001). The Jaccard coefficients for indigenous plants varied from 0.14 to 0.53, with a mean of 0.32, and apophytes varied from 0.67 to 0.94, with a mean of 0.82 (see Table 2). Among alien plants, species similarity was marginally significantly higher in archaeophytes (archaeophyte mean = 0.69, neophyte mean = 0.48, t = 2.08, p = 0.071; see Table 2).
The results show that phylogenetic diversity, assessed by the Faith’s index, was higher in the urban flora of Yekaterinburg and lower in Turinsk, as was predicted by differences in species richness. At the same time, all the considered urban floras had relatively similar MPD values (see Table 3) ranging from 297 to 312 Ma.
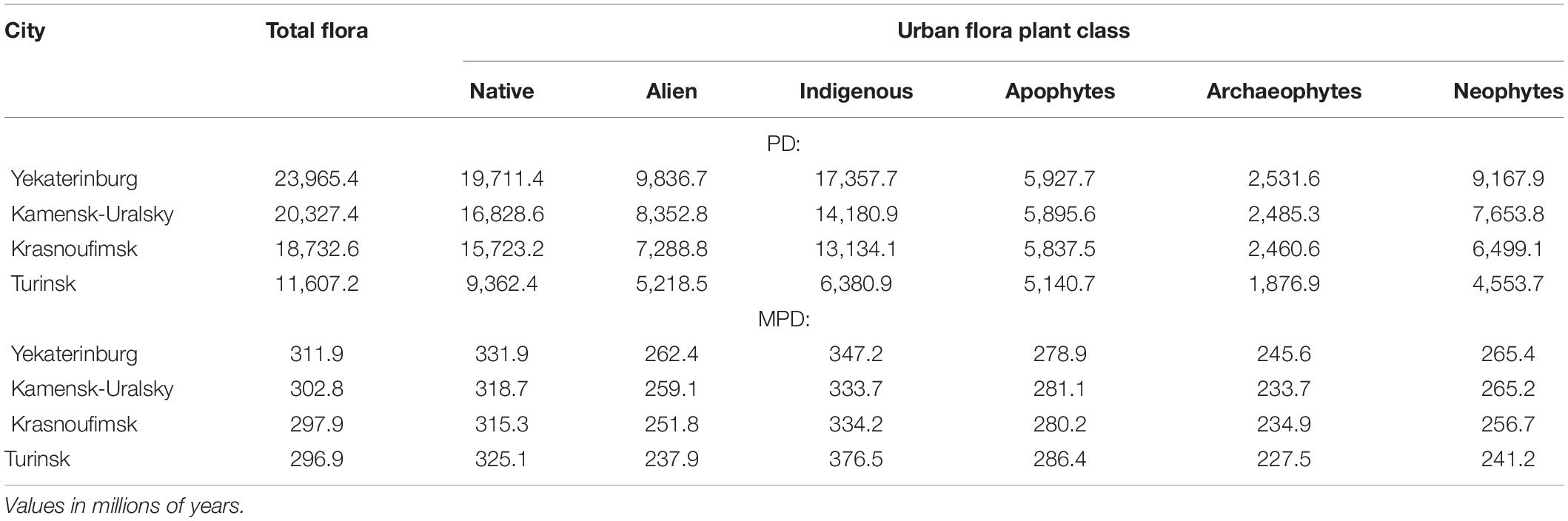
Table 3. Phylogenetic diversity (PD) and mean phylogenetic distance (MPD) of native and alien species in urban flora in the Central Urals.
The MPD of native species in all our urban floras was significantly higher than that of alien species (native mean = 322.75, alien mean = 252.80, t = 10.67, p < 0.001). The MPD of the indigenous species was significantly higher than that of apophytic (indigenous mean = 347.90, apophytic mean = 281.65, t = 6.52, p = 0.006) and alien species (alien mean = 252.80, t = 8.33, p < 0.001) while the MPD of apophytes was also significantly higher than for alien species (t = 5.078, p = 0.01), albeit with a smaller magnitude of this difference. Within the alien species, neophytes had significantly higher MPD values than archaeophytes (neophyte mean = 257.12, archaeophyte mean = 235.42, t = 3.18, p = 0.023).
In general, the MPD of all species groups increased with the city size. It was highest in the urban flora of Yekaterinburg and smallest in the urban flora of Turinsk (see Table 3) with the exception of indigenous and apophytic species MPD being highest in the flora of Turinsk (the smallest city).
SES analysis showed (Figure 3) that in all four urban floras, the MPD of native species exceeded the random expectation for MPD (i.e., SES.MPD was positive), indicating phylogenetic overdispersion, while the SES.MPD of alien species, on the contrary, was negative, and exhibited substantial phylogenetic clustering. Interestingly, the two native plant groupings behaved in fundamentally different ways: the phylogenetic overdispersion of native plants was caused by indigenous species, while clustering was apparent for apophyte species (Figure 3). Both alien species classes exhibited phylogenetic clustering (Figure 3).
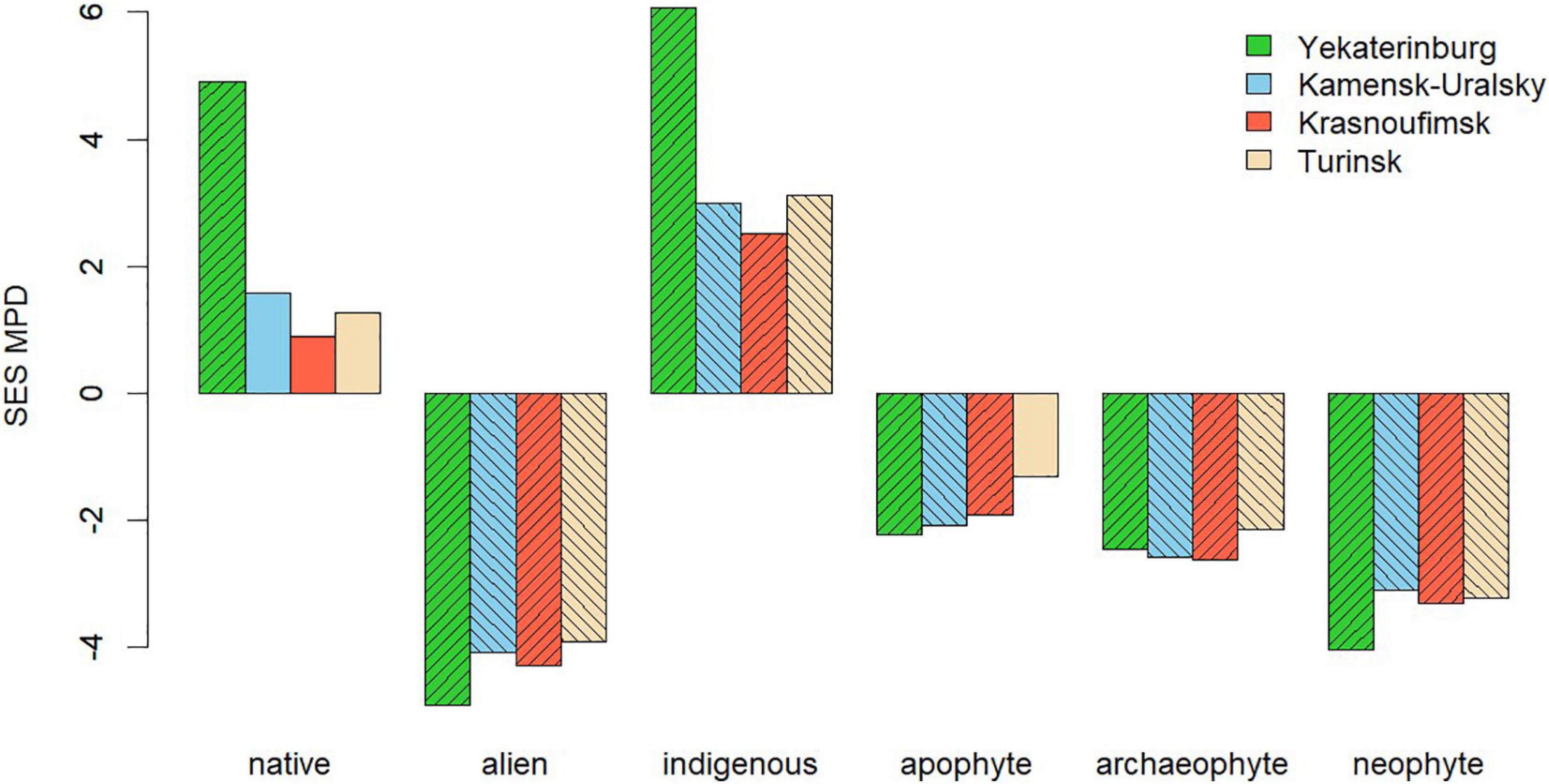
Figure 3. The results of a null-model analysis of the phylogenetic structure of urban flora classes in four cities in the Central Urals. Positive SES.MPD values correspond to phylogenetic overdispersion, and negative values correspond to phylogenetic clustering. Hatching indicates statistically significant effects (p < 0.025 for clustering and p > 0.975 for overdispersion).
Discussion
In the present study, we addressed the task of quantitative description of phylogenetic diversity and structure of urban floras of four cities in the understudied region in the Central Urals in Russia. We compared metrics of conventional taxonomic and phylogenetic diversity and also performed null-model analysis to reveal patterns of clustering and overdispersion in groups of native and alien species. Our results show that phylogenetic diversity of native species was higher than that for alien species, and the SES of MPD for natives was positive, reflecting their general dissimilarity from one another, while it was very negative for aliens, showing that they were phylogenetically clustered. However, among natives, apophytes were significantly clustered, while indigenous species were overdispersed. For the aliens, MPD was higher for archaeophytes compared to neophytes, though both groups were significantly clustered.
Our results also show that larger cities (both in terms of area and population size) support more species in total and in the various plant groupings, as has been shown previously (Pyšek, 1998; Veselkin et al., 2017; Tretyakova et al., 2018). The cities examined here harbor substantial numbers of alien taxa, more than 30% of the urban flora, which is remarkably similar to other urban plant analyses (Ricotta et al., 2009; Aronson et al., 2014; Cadotte, 2020).
Urban floras are undergoing fundamental changes, making it different from non-urban flora in diversity, composition, and structure. It is possible to identify these changes using a mix of approaches, and here we show these urban flora effects using measures of diversity, beta-diversity, and phylogenetic patterns. It is clear that species that expand their ranges in response to human activities are increasing similarity in the urban floras. Both apophytes and aliens had substantially higher Jaccard similarity values than for indigenous species, indicating that urbanization drives biotic homogenization (McKinney, 2006).
Our results show that the phylogenetic diversity of native species is higher than that of alien species, indicating that natives, and especially indigenous species, constitute a greater diversity of lineages. Among alien species, the MPD is higher in neophytes than in archaeophytes and similar observations have been made for the floras of European and American cities (Ricotta et al., 2009, 2012). More importantly, though, indigenous species were significantly overdispersed while apophytes and aliens were significantly clustered (Figure 3). These results indicate that urbanization selects for species non-randomly. An analysis of the taxonomic, ecological, and geographical diversity of the species occurring in our region may help explain our findings of phylogenetic clustering among aliens and apophytes.
The decrease in the MPD of alien species is associated with a simplification of their taxonomic structure in comparison with native species. Native species encompass all major clades of the flora of temperate latitudes (Lycopodiophyta, Polypodiophyta, Pinophyta, and Magnoliophyta). Among the alien plants, there are representatives of only two clades, Pinophyta and Magnoliophyta; spore plants are completely absent. Similarly, the decrease in MPD of apophytes in comparison with indigenous species can be explained similarly by a decrease in their taxonomic diversity. The apophytic group lacks representatives of the Lycopodiophyta, Polypodiophyta, and Pinophyta; the number of families is decreasing too; for example, representatives of the families Cyperaceae and Orchidaceae are absent.
The strong phylogenetic clustering among alien species and apophytes implies that these closely related species possess a few suites of ecological traits that allow them to adapt to certain environmental conditions and coexist in urban environments (Weiher and Keddy, 1995; Knapp et al., 2008, 2017; Proche et al., 2008; Ricotta et al., 2009) and are the outcome of phylogenetic conservatism of niches (Losos, 2008). For example, there are higher ambient temperatures in the city compared to the surroundings, which allows thermophilic plants to exist. Urban soils are highly alkaline and highly saline, which provides plants adapted to saline or high pH soils (Sukopp, 2004; Godefroid et al., 2007; Thompson and McCarthy, 2008).
Conversely to the patterns for alien species and apophytes, indigenous species were statistically significantly phylogenetically overdispersed. Indigenous species have more functional differences, can use ecological space more efficiently, and exist in a wide range of conditions (MacArthur and Levins, 1967; Proche et al., 2008). The effect of phylogenetic overdispersion is usually interpreted as an evidence for the leading role of biotic interactions leading to the competitive exclusion of closely related species and thus increasing MPD (Webb, 2000; Mayfield and Levine, 2010). We believe that this is not the case with respect to the indigenous in our urban floras. These indigenous species are associated with a set of heterogeneous habitats and there is not likely to be direct interactions between species that occur in different habitats, so it is difficult to consider the effect of competitive exclusion. Furthermore, the scale we are examining is well above that for individual interactions, and this scale should better detect the influences of the environment and habitat diversity on phylogenetic patterns (Swenson, 2019), or the fact that the natives reflect the evolutionarily diverse lineages that evolved in this region or expanded into this region after the last glaciation event, meaning that underlying biogeographic histories of speciation and migration drive local ecophylogenetic patterns (Gerhold et al., 2015).
An analysis of the ecological (habitat types) and geographical (distribution area) diversity can help explain the effect of urbanization on plant composition. Simplification of ecological diversity through habitat loss and homogenization can explain the low phylogenetic diversity of apophytes in comparison with indigenous plants, as well as archaeophytes in comparison with neophytes. Lower habitat diversity results in selection for certain traits in the apophytes in comparison to indigenous plants and is evidenced by the absence of halophytic plants, as well as rocky, petrophytic, and steppe plants. Furthermore, the apophytes appear to have larger ranges; there are no Asian, North Asian, and Ural endemic species, as well as hypoarctic-boreal, arctoboreal, and steppe species.
Similarly, archaeophytes are mostly segetal and ruderal plants (Pyšek et al., 2002) mostly from South European (Mediterranean) and Central Asian steppe regions (Pyšek et al., 2002; Ricotta et al., 2009). Conversely, neophytes are a more diverse assemblage from a wider geographic origin, including American (North American, Central American, and South American), African, East Asian, and Siberian plants with multiple avenues of introduction, and among them, there are species of meadow, steppe, coastal, halophytic, and aquatic communities. Most prominently, these neophytes include many purposefully planted ornamental species, which should come from a more diverse sampling of evolutionary lineages, thus maintaining higher phylogenetic diversity (Pearse et al., 2018), but in our findings, neophytes do not contribute to high phylogenetic diversity. Neophytes, like archaeophytes, are from disproportionately few lineages, perhaps because of not only the influences of urbanization, but also the fact that the location climate in the Ural region might limit successfully establishing species to those adapted to colder climates.
Conversely, the group of indigenous plants is highly variable both in the species number (from 71 in urban flora of Turinsk to 478 in urban flora of Yekaterinburg) and in species composition [the species similarity is 0.14–0.53 (see Tables 1, 3)]. The variability of the indigenous species group is primarily provided by the composition of natural habitats existing in the urban area. In the urban flora of Yekaterinburg, Krasnoufimsk, and Turinsk, forest plants are the richest group; the group of meadow plants is in second place for this indicator. The urban flora of Yekaterinburg contains a more diverse group of bog plants. In the urban flora of Kamensk-Uralsky, steppe species are in first place, the proportion of which increases due to groups of rocky, rocky petrophytic steppe, and petrophytic steppe plants, and forest and meadow plants are shifted to the second and third places. Urban flora of Kamensk-Uralsky is also distinguished by the presence of a large group of halophytic plants in its composition.
While we can attribute these phylogenetic patterns to the influences of urbanization, the reality is that urban environments can contain a complex mix of relictual and novel habitats (Aronson et al., 2017) that will undoubtedly select for different kinds of species. For example, land use history in urban areas can greatly impact phylogenetic diversity (Cheng et al., 2018), with relict forests containing higher phylogenetic diversity than recovered and secondary forests, which tend to be phylogenetically clustered (Borges et al., 2020). As a result, as long as urban areas have relatively few intact relict habitats, and more disturbed or other impacted sites, we should see a high prevalence of phylogenetically clustered communities. Furthermore, there are likely feedbacks between land use and plant invasion on phylogenetic patterns, especially for native communities. Gutiérrez-Cánovas et al. (2020) show that along the Spanish coast, urbanization had stronger negative impacts on native taxonomic and functional diversity when sites also contained invasive plants. We were not able to tease apart these interactions with our data, but future work should examine interactions among different drivers of biodiversity change in cities.
Finally, we wish to note the value in protecting not only indigenous species, but also the ecological diversity they represent. It is understood that the biotic homogenization caused by urban development and alien introductions serves to reduce resilience to future environmental change (McKinney, 2006). However, within cities, human populations rely on the benefits provided by healthy and diverse ecosystems (Gómez-Baggethun and Barton, 2013). Not only do alien species have the potential to directly impact ecosystem service delivery (Potgieter et al., 2017), but the loss of native diversity can also impact ecosystem services. The functioning of ecosystems has been shown to decline with both the loss of species richness (Tilman et al., 2014) and the loss of phylogenetic diversity (Cadotte, 2013). Thus, we encourage municipal policies that preserve and enhance diverse native habitats within urban areas.
Conclusion
The urban flora is characterized by high alien species richness, but relatively low phylogenetic diversity. This reflects the fact that natives represent high taxonomic, geographic, and ecological differentiation and that they persist in a broad set ecological conditions and provide diverse benefits. The alien species are not a random set of species; they include taxonomically closely related species with similar ecological properties that might provide adaptation to urban environmental conditions. Thus, the high species richness of the urban flora of the Ural cities, as well as European ones (Knapp et al., 2008), is provided mainly by closely related species that are functionally similar and adapted to an urban environment.
Data Availability Statement
The original contributions presented in the study are available in the GBIF repository (https://www.gbif.org/dataset/79a2fbbd-a1fc-4f1f-a5af-9c3a6ad398db).
Author Contributions
AT, BY, and MC conceived this work and designed the methodology. AT, BY, PK, and NG collected and analyzed the data. AT and BY created the original draft. MC supervised the analysis and writing process. All authors edited and revised subsequent drafts of the manuscript, approved the final version, and agreed to be held accountable for the work.
Funding
Funding for this collaboration was provided to MC by the Connaught Global Challenges Award, the Office of the Vice-President International, the School of Graduate Studies at the University of Toronto, the Office of the Vice-Principal Research at the University of Toronto Scarborough, and funding from the Natural Sciences and Engineering Research Council of Canada (#386151). This work was supported in part by the Program for Improving the Competitiveness of the Ural Federal University (the decree no. 211 of the Government of the Russian Federation, contract no. 02.A03.21.0006) and by the Russian Foundation for Basic Research (project no. 19-04-01084).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Antipova, S. V., and Antipova, E. M. (2016). Urbanoflora of the city of Krasnoyarsk (vascular plants). Krasnoyarsk: Krasnoyarsk State Pedagogical University.
Aronson, M. F., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., et al. (2014). A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 281:20133330. doi: 10.1098/rspb.2013.3330
Aronson, M. F., Lepczyk, C. A., Evans, K. L., Goddard, M. A., Lerman, S. B., MacIvor, J. S., et al. (2017). Biodiversity in the city: key challenges for urban green space management. Front. Ecol. Environ. 15:189–196. doi: 10.1002/fee.1480
Aronson, M. F., Nilon, C. H., Lepczyk, C. A., Parker, T. S., Warren, P. S., Cilliers, S. S., et al. (2016). Hierarchical filters determine community assembly of urban species pools. Ecology 97, 2952–2963. doi: 10.1002/ecy.1535
Baranova, O. G., Shcherbakov, A. V., Senator, S. A., Panasenko, N. N., Sagalayev, V. A., and Saksonov, S. V. (2018). The main terms and concepts used in the study of alien and synanthropic flora. Phytodivers. East. Eur. 12, 4–22. doi: 10.24411/2072-8816-2018-10031
Beresutsky, M. A., and Panin, A. V. (2007). Urban flora: structure and tendencies of anthropogenous dynamics. Botanicheskii Zhurnal 92, 1481–1489.
Bitomský, M., Mládková, P., Pakeman, R. J., and Duchoslav, M. (2020). Clade composition of a plant community indicates its phylogenetic diversity. Ecol. Evol. 10, 3747–3757. doi: 10.1002/ece3.6170
Borges, E. R., Dexter, K. G., Bueno, M. L., Pontara, V., and Carvalho, F. A. (2020). The evolutionary diversity of urban forests depends on their land-use history. Urban Ecosyst. 23, 631–643. doi: 10.1007/s11252-020-00938-y
Cadotte, M. W. (2013). Experimental evidence that evolutionarily diverse assemblages result in higher productivity. Proc. Natl. Acad. Sci. U. S. A. 110, 8996–9000. doi: 10.1073/pnas.1301685110
Cadotte, M. W. (2017). Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 20, 989–996. doi: 10.1111/ele.12796
Cadotte, M. W. (2020). The list of vascular plants for the city of Toronto. Ecol. Solu. Evid. 2, e12036.
Cadotte, M. W., Carboni, M., Si, X., and Tatsumi, S. (2019). Do traits and phylogeny support congruent community diversity patterns and assembly inferences? J. Ecol. 107, 2065–2077. doi: 10.1111/1365-2745.13247
Cadotte, M. W., and Davies, T. J. (2016). Phylogenies in Ecology: A Guide to Concepts and Methods. Princeton: Princeton University Press.
Cadotte, M. W., and Tucker, C. M. (2017). Should Environmental Filtering be Abandoned? Trends Ecol. Evol. 32, 429–437. doi: 10.1016/j.tree.2017.03.004
Cadotte, M. W., Yasui, S. L. E., Livingstone, S., and MacIvor, J. S. (2017). Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol. Invasions 19, 3489–3503. doi: 10.1007/s10530-017-1586-y
Cavender-Bares, J., Kozak, K. H., Fine, P. V. A., and Kembel, S. W. (2009). The merging of community ecology and phylogenetic biology. Ecol. Lett. 12, 693–715. doi: 10.1111/j.1461-0248.2009.01314.x
Čeplová, N., Lososová, Z., Zelený, D., Chytrý, M., Danihelka, J., Fajmon, K., et al. (2015). Phylogenetic diversity of central-European urban plant communities: effects of alien species and habitat types. Preslia 87, 1–16.
Cheng, X.-L., Yuan, L.-X., Nizamani, M. M., Zhu, Z.-X., Friedman, C. R., and Wang, H.-F. (2018). Taxonomic and phylogenetic diversity of vascular plants at Ma’anling volcano urban park in tropical Haikou. China: Reponses to soil properties. PLoS One 13:e0198517. doi: 10.1371/journal.pone.0198517
Faith, D. P. (1992). Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10. doi: 10.1016/0006-3207(92)91201-3
Flynn, D. F. B., Mirotchnick, N., Jain, M., Palmer, M. I., and Naeem, S. (2011). Functional and phylogenetic diversity as predictors of biodiversity-ecosystem funciton relationships. Ecology 92, 1573–1581. doi: 10.1890/10-1245.1
Gerhold, P., Cahill, J. F., Winter, M. I, Bartish, V., and Prinzing, A. (2015). Phylogenetic patterns are not proxies of community assembly mechanisms (they are far better). Funct. Ecol. 29, 600–614. doi: 10.1111/1365-2435.12425
Godefroid, S., Monbaliu, D., and Koedam, N. (2007). The role of soil and microclimatic variables in the distribution patterns of urban wasteland flora in Brussels, Belgium. Landsc. Urban Plan. 80, 45–55. doi: 10.1016/j.landurbplan.2006.06.001
Golovanov, Ya. M, and Abramova, L. M. (2017). Comparative analysis of urban floras structure in the southern industrial zone of the Cis-Urals. Botanicheskii Zhurnal 102, 540–562.
Gómez-Baggethun, E., and Barton, D. N. (2013). Classifying and valuing ecosystem services for urban planning. Ecol. Econ. 86, 235–245. doi: 10.1016/j.ecolecon.2012.08.019
Gutiérrez-Cánovas, C., Sánchez-Fernández, D., González-Moreno, P., Mateos-Naranjo, E., Castro-Díez, P., and Vilà, M. (2020). Combined effects of land-use intensification and plant invasion on native communities. Oecologia 192, 823–836. doi: 10.1007/s00442-020-04603-1
Kembel, S. W., Cowan, P. D., Helmus, M. R., Cornwell, W. K., Morlon, H., Ackerly, D. D., et al. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464. doi: 10.1093/bioinformatics/btq166
Khokhryakov, A. P. (2000). Taxonomic spectra and their role in comparative floristics. Botanicheskii Zhurnal 85, 1–11.
Knapp, S., Kühn, I., Schweiger, O., and Klotz, S. (2008). Challenging Urban Species Diversity: contrasting Phylogenetic Patterns across Plant Functional Groups in Germany. Ecol. Lett. 11, 1054–1064. doi: 10.1111/j.1461-0248.2008.01217.x
Knapp, S., Winter, M., and Klotz, S. (2017). Increasing species richness but decreasing phylogenetic richness and divergence over a 320-year period of urbanization. J. Appl. Ecol. 54, 1152–1160. doi: 10.1111/1365-2664.12826
Knyazev, M. S., Chkalov, A. V., Tretyakova, A. S., Zolotareva, N. V., Podgaevskaya, E. N., Pakina, D. V., et al. (2019a). Annotated checklist of the flora of Sverdlovsk region. Part V: dicotyledonous plants (Rosaceae). Phytodivers. East. Eur. 13, 305–352. doi: 10.24411/2072-8816-2019-10056
Knyazev, M. S., Tretyakova, A. S., Podgaevskaya, E. N., Zolotareva, N. V., and Kulikov, P. V. (2019b). Annotated checklist of the flora of Sverdlovsk region. Part IV: dicotyledonous plants (Empetraceae-Droseraceae). Phytodivers. East. Eur. 13, 130–196. doi: 10.24411/2072-8816-2019-10046
Knyazev, M. S., Podgaevskaya, E. N., Tretyakova, A. S., Zolotareva, N. V., and Kulikov, P. V. (2020). Annotated checklist of the flora of Sverdlovsk Region. Part VI: dicotyledonous plants (Fabaceae-Lobeliaceae). Phytodivers. East. Eur. 14, 190–331. doi: 10.24411/2072-8816-2020-10077
Knyazev, M. S., Tretyakova, A. S., Podgaevskaya, E. N., Zolotareva, N. V., and Kulikov, P. V. (2017). An annotated checklist of the flora of Sverdlovsk’s Region. Part II: monocotyledonous plants. Phytodivers. East. Eur. 11, 4–108.
Knyazev, M. S., Tretyakova, A. S., Podgaevskaya, E. N., Zolotareva, N. V., and Kulikov, P. V. (2018). An annotated checklist of the flora of Sverdlovsk’s Region. Part III: dicotyledonous plants (Aristolochiaceae-Monotropaceae). Phytodivers. East. Eur. 12, 6–101. doi: 10.24411/2072-8816-2018-10013
Knyazev, M. S., Zolotareva, N. V., Podgaevskaya, E. N., Tretyakova, A. S., and Kulikov, P. V. (2016). An annotated checklist of the flora of Sverdlovsk’s region. Part I: spore and Gymnosperms plants. Phytodivers. East. Eur. 10, 11–41.
Kueffer, C. (2017). Plant invasions in the Anthropocene. Science 358, 724–725. doi: 10.1126/science.aao6371
La Sorte, F., McKinney, M. L., and Pyšek, P. (2007). Compositional similarity among urban floras within and across continents: biogeographical consequences of human-mediated biotic interchange. Glob. Change Biol. 13, 913–921. doi: 10.1111/j.1365-2486.2007.01329.x
Lonsdale, W. M. (1999). Global patterns of plant invasions and the concept of invasibility. Ecology 80, 1522–1536. doi: 10.2307/176544
Losos, J. B. (2008). Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995–1003. doi: 10.1111/j.1461-0248.2008.01229.x
Lososová, Z., Čeplová, N., Chytrý, M., Tichý, L., Danihelka, J., Fajmon, K., et al. (2016). Is phylogenetic diversity a good proxy for functional diversity of plant communities? A case study from urban habitats. J. Veg. Sci. 27, 1036–1046. doi: 10.1111/jvs.12414
MacArthur, R., and Levins, R. (1967). The Limiting Similarity, Convergence, and Divergence of Coexisting Species. Am. Nat. 101, 377–385. doi: 10.1086/282505
Mayfield, M. M., and Levine, J. M. (2010). Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 13, 1085–1093. doi: 10.1111/j.1461-0248.2010.01509.x
McKinney, M. L. (2006). Urbanization as a major cause of biotic homogenization. Biol. Conserv. 127, 247–260. doi: 10.1016/j.biocon.2005.09.005
McKinney, M. L., and Lockwood, J. L. (1999). Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453. doi: 10.1016/S0169-5347(99)01679-1
Olden, J. D., Poff, N. L. R., Douglas, M. R., Douglas, M. E., and Fausch, K. D. (2004). Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19, 18–24. doi: 10.1016/j.tree.2003.09.010
Pearse, W. D., Cavender-Bares, J., Hobbie, S. E., Avolio, M. L., Bettez, N., and Chowdhury, R. Roy, et al. (2018). Homogenization of plant diversity, composition, and structure in North American urban yards. Ecosphere 9:e02105. doi: 10.1002/ecs2.2105
Potgieter, L. J., and Cadotte, M. W. (2020). The application of selected invasion frameworks to urban ecosystems. NeoBiota 62, 365–386. doi: 10.3897/neobiota.62.50661
Potgieter, L. J., Gaertner, M., Kueffer, C., Larson, B. M., Livingstone, S. W., O’Farrell, P. J., et al. (2017). Alien plants as mediators of ecosystem services and disservices in urban systems: a global review. Biol. Invasions 19, 3571–3588. doi: 10.1007/s10530-017-1589-8
Proche, S., Wilson, J. R. U., Richardson, D. M., and Rejmánek, M. (2008). Searching for phylogenetic pattern in biological invasions. Glob. Ecol. Biogeogr. 17, 5–10. doi: 10.1111/j.1466-8238.2007.00333.x
Pyšek, P. (1998). Alien and native species in Central European urban floras: a quantitative comparison. J. Biogeogr. 25, 155–163. doi: 10.1046/j.1365-2699.1998.251177.x
Pysek, P., Richardson, D. M., and Williamson, M. (2004). Predicting and explaining plant invasions through analysis of source area floras: some critical considerations. Divers. Distrib. 10, 179–187. doi: 10.1111/j.1366-9516.2004.00079.x
Pyšek, P., Sádlo, J., and Mandák, B. (2002). Catalogue of alien plants of the Czech Republic. Preslia 74, 97–186.
R Core Team. (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ricotta, C., La Sorte, F. A., Pysek, P., Rapson, G. L., Celesti-Grapow, L., and Thompson, K. (2009). Phyloecology of urban alien floras. J. Ecol. 97, 1243–1251. doi: 10.1111/j.1365-2745.2009.01548.x
Ricotta, C., La Sorte, F. A., Pyšek, P., Rapson, G. L., Celesti-Grapow, L., and Thompson, K. (2012). Phylogenetic beta diversity of native and alien species in European urban floras. Glob. Ecol. Biogeogr. 21, 751–759. doi: 10.1111/j.1466-8238.2011.00715.x
Senator, S. A., Saksonov, S. V., Rakov, N. S., Vasyukov, V. M., Ivanova, A. V., and Sidyakina, L. V. (2015). Vascular plants of Togliatti and its surrounding (Samara region). Phytodivers. East. Eur. 9, 32–101.
Sukopp, H. (2004). Human-Caused Impact on Preserved Vegetation. Landsc. Urban Plan. 68, 347–355. doi: 10.1016/S0169-2046(03)00152-X
Swenson, N. G. (2019). Phylogenetic Ecology: A History, Critique, and Remodeling. Chicago: University of Chicago Press.
Thompson, K., and McCarthy, M. A. (2008). Traits of British alien and native urban plants. J. Ecol. 96, 853–859. doi: 10.1111/j.1365-2745.2008.01383.x
Tilman, D., Isbell, F., and Cowles, J. M. (2014). Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. doi: 10.1146/annurev-ecolsys-120213-091917
Tretyakova, A. S. (2014). Distribution of plant species composition in natural and anthropogenic habitats of Ekaterinburg city. Botanicheskii Zhurnal 99, 1277–1282.
Tretyakova, A. S. (2016). Characteristics taxonomic structure flora urban areas of the Middle Urals (Sverdlovsk region). Samara J. Sci. 1, 66–71. doi: 10.17816/snv20161114
Tretyakova, A. S., and Shurova, E. A. (2013). The flora of Yekaterinburg city. Botanicheskii Zhurnal 98, 210–219.
Tretyakova, A. S., Veselkin, D. V., Senator, S. A., and Golovanov, Ya.M (2018). Factors of Richness of Urban Floras in the Ural-Volga Region. Russ. J. Ecol. 49, 201–208. doi: 10.1134/S1067413618030098
Tucker, C. M., Cadotte, M. W., Carvalho, S. B., Davies, T. J., Ferrier, S., Fritz, S. A., et al. (2017). A guide to phylogenetic metrics for conservation, community ecology and macroecology. Biol. Rev. 92, 698–715. doi: 10.1111/brv.12252
Tucker, C. M., Davies, T. J., Cadotte, M. W., and Pearse, W. D. (2018). On the relationship between phylogenetic diversity and trait diversity. Ecology 99, 1473–1479. doi: 10.1002/ecy.2349
Veselkin, D. V., Tretyakova, A. S., Senator, S. A., Saksonov, S. V., Mukhin, V. A., and Rozenberg, G. S. (2017). Geographical factors of the abundance of flora in Russian cities. Dokl. Earth Sci. 476, 1113–1115. doi: 10.1134/S1028334X1709029X
von Ledebour, C. F. (1842-1853). Flora Rossica Sive Enumeratio Plantarum in Totius Imperii Rossici Provinciis Europaeis, Asiaticis et Americanis Hucusque Observatarum. Stuttgartiae: Sumtibus Librariae E. Schweizerbart. doi: 10.1134/s1028334x1709029x
Webb, C. O. (2000). Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Soc. Nat. 156, 145–155. doi: 10.1086/303378
Webb, C. O., Ackerly, D. D., McPeek, M. A., and Donoghue, M. J. (2002). Phylogenies and Community Ecology. Annu. Rev. Ecol. Syst. 33, 475–505. doi: 10.1146/annurev.ecolsys.33.010802.150448
Weiher, E., and Keddy, P. A. (1995). The assembly of experimental wetland plant communities. Oikos 73, 323–335. doi: 10.2307/3545956
Zanne, A. E., Tank, D. C., Cornwell, W. K., Eastman, J. M., Smith, S. A., Fitz, J. R. G., et al. (2014). Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92. doi: 10.1038/nature12872
Keywords: alien plants, archaeophyte, mean phylogenetic distance, native plants, neophyte, null-model analysis, phylogenetic diversity
Citation: Tretyakova AS, Yakimov BN, Kondratkov PV, Grudanov NY and Cadotte MW (2021) Phylogenetic Diversity of Urban Floras in the Central Urals. Front. Ecol. Evol. 9:663244. doi: 10.3389/fevo.2021.663244
Received: 02 February 2021; Accepted: 08 July 2021;
Published: 04 August 2021.
Edited by:
Jason Munshi-South, Fordham University, United StatesReviewed by:
Jennifer Weber, Southern Illinois University Carbondale, United StatesLauren Frazee, Rutgers University, Newark, United States
Copyright © 2021 Tretyakova, Yakimov, Kondratkov, Grudanov and Cadotte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basil N. Yakimov, damselfly@yandex.ru
 Alyona S. Tretyakova
Alyona S. Tretyakova Basil N. Yakimov
Basil N. Yakimov Pavel V. Kondratkov
Pavel V. Kondratkov Nickolay Yu. Grudanov
Nickolay Yu. Grudanov Marc W. Cadotte
Marc W. Cadotte