Abstract
Purpose
Caloric restriction (CR) and Roux-en-Y Gastric Bypass (RYGB) are considered effective means of body weight control, but the mechanism by which CR and RYGB protect against high-fat diet (HFD)-induced obesity remains elusive. The browning of white adipose tissue (WAT) is a potential approach to combat obesity. Here we assess whether browning of WAT is involved in CR- and RYGB-treatment.
Methods
The average size of adipocytes was determined by histological analysis. Expression of thermogenic genes in both human subjects and mice were measured by quantitative real-time PCR and immunohistochemical staining.
Results
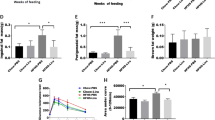
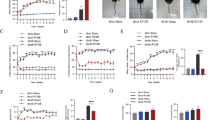
The average size of adipocytes was bigger, while the expression of thermogenic genes such as uncoupling protein 1 (UCP1), nuclear factor erythroid-2 like 1 (NRF1) and PPARγ coactivator-1 α (PGC1α) were lower in the WAT of obese subjects when compared to lean controls. Both CR and RYGB promoted weight and fat loss. Increment of the average adipocytes size and down-regulation of thermogenic genes were significantly reversed by both CR and RYGB in the WAT of obese mice.
Conclusions
Our findings showed that CR and RYGB significantly improved high-fat diet-induced lipid accumulation by promoting the browning of WAT.





Similar content being viewed by others
Data availability
All data relevant to the study are included in the article or uploaded as supplemental information.
Code availability
Not applicable.
Abbreviations
- CR:
-
Caloric restriction
- RYGB:
-
Roux-en-Y Gastric Bypass
- WAT:
-
White adipose tissue
- BAT:
-
Brown adipose tissue
- UCP1:
-
Uncoupling protein 1
- NRF1:
-
Nuclear factor erythroid-2 like-1
- PGC1α:
-
PPARγ coactivator-1 α
References
WHO (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894:1–8 (1–253)
Haslam DW, James WPT (2005) Obesity. Lancet 366:1197–1209. https://doi.org/10.1016/S0140-6736(05)67483-1
Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC et al (2012) Health benefits of gastric bypass surgery After 6 years. JAMA-J Am Med Assoc 308:1122–1131. https://doi.org/10.1001/2012.jama.11164
Klempel MC, Kroeger CM, Bhutani S, Trepanowski JF, Varady KA (2012) Intermittent fasting combined with calorie restriction is effective for weight loss and cardio-protection in obese women. Nutr J 11:98. https://doi.org/10.1186/1475-2891-11-98
Most J, Tosti V, Redman LM, Fontana L (2017) Calorie restriction in humans: an update. Ageing Res Rev 39:36–45. https://doi.org/10.1016/j.arr.2016.08.005
Kushner RF (2018) Weight loss strategies for treatment of obesity: lifestyle management and pharmacotherapy. Prog Cardiovasc Dis 61:246–252. https://doi.org/10.1016/j.pcad.2018.06.001
Lettieri-Barbato D, Giovannetti E, Aquilano K (2016) Effects of dietary restriction on adipose mass and biomarkers of healthy aging in human. Aging-Us 8:3341–3355. https://doi.org/10.18632/aging.101122
Neinast MD, Frank AP, Zechner JF, Li Q, Vishvanath L, Palmer BF et al (2015) Activation of natriuretic peptides and the sympathetic nervous system following Roux-en-Y gastric bypass is associated with gonadal adipose tissues browning. Mol Metab 4:427–436. https://doi.org/10.1016/j.molmet.2015.02.006
Ali AT, Hochfeld WE, Myburgh R, Pepper MS (2013) Adipocyte and adipogenesis. Eur J Cell Biol 92:229–236. https://doi.org/10.1016/j.ejcb.2013.06.001
Cinti S (2009) Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol-Endocrinol Metab 297:E977–E986. https://doi.org/10.1152/ajpendo.00183.2009
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang A-H et al (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. https://doi.org/10.1016/j.cell.2012.05.016
Sidossis L, Kajimura S (2015) Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Investig 125:478–486. https://doi.org/10.1172/JCI78362
Kalinovich AV, de Jong JMA, Cannon B, Nedergaard J (2017) UCP1 in adipose tissues: two steps to full browning. Biochimie 134:127–137. https://doi.org/10.1016/j.biochi.2017.01.007
Li D, Li S, Pan Q, Zhai H, Peng M, Wang X et al (2018) Gastric mammalian target of rapamycin signaling contributes to inhibition of ghrelin expression induced by Roux-En-Y Gastric Bypass. Cell Physiol Biochem 51:664–680. https://doi.org/10.1159/000495325
Zhai H, Li Z, Peng M, Huang Z, Qin T, Chen L et al (2018) Takeda G protein-coupled receptor 5-mechanistic target of rapamycin complex 1 signaling contributes to the increment of glucagon-like peptide-1 production after Roux-en-Y Gastric Bypass. EBioMedicine 32:201–214. https://doi.org/10.1016/j.ebiom.2018.05.026
Pan Q, Qin T, Gao Y, Li S, Li D, Peng M et al (2019) Hepatic mTOR-AKT2-Insig2 signaling pathway contributes to the improvement of hepatic steatosis after Roux-en-Y Gastric Bypass in mice. BBA-Mol Basis Dis 1865:525–534. https://doi.org/10.1016/j.bbadis.2018.12.014
Nedergaard J, Cannon B (2014) The browning of white adipose tissue: some burning issues. Cell Metab 20:396–407. https://doi.org/10.1016/j.cmet.2014.07.005
Inagaki T, Sakai J, Kajimura S (2016) Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat Rev Mol Cell Biol 17:480–495. https://doi.org/10.1038/nrm.2016.62
Thompson D, Karpe F, Lafontan M, Frayn K (2012) Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev 92:157–191. https://doi.org/10.1152/physrev.00012.2011
Coelho M, Oliveira T, Fernandes R (2013) Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci 9:191–200. https://doi.org/10.5114/aoms.2013.33181
Kajimura S, Saito M (2014) A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 76:225–249. https://doi.org/10.1146/annurev-physiol-021113-170252
Thyagarajan B, Foster MT (2017) Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Investig. https://doi.org/10.1515/hmbci-2017-0016
Barquissau V, Beuzelin D, Pisani DF, Beranger GE, Mairal A, Montagner A et al (2016) White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol Metab 5:352–365. https://doi.org/10.1016/j.molmet.2016.03.002
Sun Y, Wang R, Zhao S, Li W, Liu W, Tang L et al (2019) FGF9 inhibits browning program of white adipocytes and associates with human obesity. J Mol Endocrinol 62:79–90. https://doi.org/10.1530/JME-18-0151
Maurer S, Harms M, Boucher J (2020) The colorful versatility of adipocytes: white-to-brown transdifferentiation and its therapeutic potential in man. FEBS J. https://doi.org/10.1111/febs.15470
Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A et al (2015) Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84:202–211. https://doi.org/10.1016/j.yjmcc.2015.05.002
Qian S-W, Tang Y, Li X, Liu Y, Zhang Y-Y, Huang H-Y et al (2013) BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA 110:E798–E807. https://doi.org/10.1073/pnas.1215236110
Tseng Y-H, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000-U44. https://doi.org/10.1038/nature07221
Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al (2012) FGF21 regulates PGC-1 alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26:271–81. https://doi.org/10.1101/gad.177857.111
Lizcano F (2019) The beige adipocyte as a therapy for metabolic diseases. Int J Mol Sci 20:5058. https://doi.org/10.3390/ijms20205058
Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S et al (2014) Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63:4089–99. https://doi.org/10.2337/db14-0746
Wang C-H, Lundh M, Fu A, Kriszt R, Huang TL, Lynes MD et al (2020) CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci Transl Med 12:eaaz8664. https://doi.org/10.1126/scitranslmed.aaz8664
Shen H, Jiang L, Lin JD, Omary MB, Rui L (2019) Brown fat activation mitigates alcohol-induced liver steatosis and injury in mice. J Clin Investig 129:2305–2317. https://doi.org/10.1172/JCI124376
Fontana L, Partridge L, Longo VD (2010) Extending healthy life span—from yeast to humans. Science 328:321–326. https://doi.org/10.1126/science.1172539
Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K et al (2010) Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1:e10. https://doi.org/10.1038/cddis.2009.8
La Russa D, Marrone A, Mandala M, Macirella R, Pellegrino D (2020) Antioxidant/anti-inflammatory effects of caloric restriction in an aged and obese rat model: the role of adiponectin. Biomedicines 8:532. https://doi.org/10.3390/biomedicines8120532
Khalafi M, Symonds ME, Akbari A (2021) The impact of exercise training versus caloric restriction on inflammation markers: a systemic review and meta-analysis. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.1873732
Reinisch I, Schreiber R, Prokesch A (2020) Regulation of thermogenic adipocytes during fasting and cold. Mol Cell Endocrinol 512:110869. https://doi.org/10.1016/j.mce.2020.110869
Bielefeldt K (2014) Bariatric surgery versus intensive medical therapy for diabetes. N Engl J Med 371:681–2. https://doi.org/10.1056/NEJMc1407393
Rachid B, van de Sande-Lee S, Rodovalho S, Folli F, Beltramini GC, Morari J et al (2015) Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. Int J Obes 39:1515–22. https://doi.org/10.1038/ijo.2015.94
Lynch L, Hogan AE, Duquette D, Lester C, Banks A, LeClair K et al (2016) iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab 24:510–9. https://doi.org/10.1016/j.cmet.2016.08.003
Funding
This work was supported by grants from the National Natural Science Foundation of China (81770794, 314010010), the Fundamental Research Funds for the Central Universities (21620423).
Author information
Authors and Affiliations
Contributions
GX designed research; DH, ZZ, RL and JH performed research; GX, ZD analyzed data; GX, ZD, DH and ZZ wrote and edited the paper. All authors contributed to the discussion and revised the article and all approved the final versions of the manuscript. GX is responsible for the integrity of the work as a whole.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical statement
Participation in this study was voluntary and written informed consent was obtained from each participant. The guidelines of the Declaration of Helsinki of the World Medical Association were followed. All protocols were approved by the Research Ethics Committee of the First Affiliated Hospital of Jinan University.
Research involving human participants and/or animals
Animals used in this study were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 8023, revised 1978). All animal protocols were approved by the Animal Care and Use Committee of Jinan University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1 and 2.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, D., Zhang, Z., Dong, Z. et al. Caloric restriction and Roux-en-Y Gastric Bypass promote white adipose tissue browning in mice. J Endocrinol Invest 45, 139–148 (2022). https://doi.org/10.1007/s40618-021-01626-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-021-01626-0