Abstract
Background
The assessment of liver regeneration is particularly critical for patients with acute-on-chronic liver failure (ACLF). Exosome has both the advantages of specificity of liver biopsy and noninvasion of peripheral blood, which may be the potential biomarker of liver disease.
Methods
The patients with chronic hepatitis B (CHB) and ACLF were enrolled from outpatients and inpatients in Beijing Youan Hospital, Capital Medical University. The exosomes in plasma were extracted by ultracentrifuge using Optima XPN-100 Ultracentrifuge. Exosomes were dyed with fluorescent direct-labeled antibody and the expression profile was assayed using ImageStream® X MKII Imaging Flow Cytometer.
Results
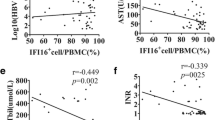
The percentage of exosomes with ALB and CD63 was significant higher in ACLF than that in CHB. The percentage of exosomes with ALB and CD63 and VEGF increased in CHB, but decreased in ACLF. The exosomes with ALB, CD63, and VEGF were significant more in survival group than that in dead group in patients with ACLF. The sensitivity and specificity of exosomes with CD63, ALB, and VEGF were significantly higher than the other markers of liver regeneration and prognostic valuation in patients with ACLF including AFP. The hepatocyte-derived exosomes expression profile had no difference in different stages and different AFP levels of patients with ACLF.
Conclusion
The exosomes profile with ALB and VEGF may be a more accurate and specific biomarker of liver regeneration and prognostic valuation than AFP in patients with ACLF. In addition, the exosomes profile with CD63 and ALB may be an early-warning marker in patients with ACLF.





Similar content being viewed by others
References
Tang CM, Yau TO, Yu J. Management of chronic hepatitis B infection:current treatment guidelines, challenges, and new developments. World J Gastroenterol 2014;20:6262–6278
Liver Failure and Artificial Liver Disease Group, Society of Infectious Diseases, Serious Liver Disease and Artificial Liver Disease Group, Society of Hepatology, Chinese Medical Association. Guidelines for diagnosis and treatment of liver failure (2012 version). Chin J Exp Clin Infect Dis 2012;5:321–327
Sarin SK, Kedarisetty CK, Abbas Z, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol Int 2014;8:453–471
Liver Failure and Artificial Liver Disease Group, Society of Infectious Diseases, Serious Liver Disease and Artificial Liver Disease Group, Society of Hepatology, Chinese Medical Association. Guidelines for diagnosis and treatment of liver failure (2018 version). J Clin Hepatobiliary Dis 2019;5:38–44
Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol 2016;13:131–149
Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, Noreña-Herrera C, Vidaña-Perez D, Delgado-Sanchez G, Uribe M, Barrientos-Gutierrez T. Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol 2015;14:631–641
Schiødt FV, Ostapowicz G, Murray N, Satyanarana R, Zaman A, Munoz S, Lee WM. Alpha-fetoprotein and prognosis in acute liver failure. Liver Transpl 2006;12:1776–1781
Lemoinne S, Thabut D, Housset C, Moreau R, Valla D, Boulanger CM, Rautou PE. The emerging roles of microvesicles in liver diseases. Nat Rev Gastroenterol Hepatol 2014;11:350–361
Lambrecht J, Verhulst S, Mannaerts I, Reynaert H, van Grunsven LA. Prospects in non-invasive assessment of liver fibrosis: Liquid biopsy as the future gold standard? Biochim Biophys Acta Mol Basis Dis 2018;1864:1024–1036
Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol 2016;65:213–221
Lai RC, Yeo RW, Tan KH, Lim SK. Exosomes for drug delivery: a novel application for the mesenchymal stem cell. Biotechnol Adv 2013;31:543–551
Sun F, Wang JZ, Luo JJ, Wang YQ, Pan Q. Exosomes in the oncobiology, diagnosis, and therapy of hepatic carcinoma: a new player of an old game. Biomed Res Int 2018;25:2747461
Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology 2016;64:2219–2233
Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol 2016;64:60–68
Malato Y, Naqvi S, Schürmann N, Ng R, Wang B, Zape J, Kay MA, Grimm D, Willenbring H. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest 2011;121:4850–4860
Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012;13:e00049
Payancé A, Silva-Junior G, Bissonnette J, Tanguy M, Pasquet B, Levi C, Roux O, Nekachtali O, Baiges A, Hernández-Gea V, Laouénan C, Lebrec D, Albuquerque M, Paradis V, Moreau R, Valla D, Durand F, Boulanger CM, Garcia-Pagan JC, Rautou PE. Hepatocyte microvesicle levels improve prediction of mortality in patients with cirrhosis. Hepatology 2018;68:1508–1518
Eguchi A, Feldstein AE. Extracellular vesicles in non-alcoholic and alcoholic fatty liver diseases. Liver Res 2018;2:30–34
Balaphas A, Meyer J, Sadoul R, Morel P, Gonelle-Gispert C, Bühler LH. Extracellular vesicles: future diagnostic and therapeutic tools for liver disease and regeneration. Liver Int 2019;39:1801–1817
Urban SK, Mocan T, Sänger H, Lukacs-Kornek V, Kornek M. Extracellular vesicles in liver diseases: diagnostic, prognostic, and therapeutic application. Semin Liver Dis 2019;39:70–77
Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One 2014;9:e113651
Murakami Y, Toyoda H, Tanahashi T, Tanaka J, Kumada T, Yoshioka Y, Kosaka N, Ochiya T, Taguchi YH. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PLoS One 2012;7:e48366
Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, Gores GJ, Shah VH. Alcohol stimulates macrophage activation through caspase dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 2016;64:651–660
Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, Schuppan D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology 2012;143:448–458
Lannigan J, Erdbruegger U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods 2017;112:55–67
Cho YE, Im EJ, Moon PG, Mezey E, Song BJ, Baek MC. Increased liver-specific proteins in circulating extracellular vesicles as potential biomarkers for drug- and alcohol-induced liver injury. PLoS One 2017;12:e0172463
Sancho-Bru P, Altamirano J, Rodrigo-Torres D, Coll M, Millán C, José Lozano J, Miquel R, Arroyo V, Caballería J, Ginès P, Bataller R. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 2012;55:1931–1941
Bissonnette J, Altamirano J, Devue C, Roux O, Payancé A, Lebrec D, Bedossa P, Valla D, Durand F, Ait-Oufella H, Sancho-Bru P, Caballeria J, Ginès P, Boulanger CM, Bataller R, Rautou PE. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 2017;66:555–563
Lemoinne S, Cadoret A, Rautou PE, El Mourabit H, Ratziu V, Corpechot C, Rey C, Bosselut N, Barbu V, Wendum D, Feldmann G, Boulanger C, Henegar C, Housset C, Thabut D. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 2015;61:1041–1055
Wang X, Kwak KJ, Yang Z, Zhang A, Zhang X, Sullivan R, Lin D, Lee RL, Castro C, Ghoshal K, Schmidt C, Lee LJ. Extracellular mRNA detected by molecular beacons in tethered lipoplex nanoparticles for diagnosis of human hepatocellular carcinoma. PLoS One 2018;13:e018552
Funding
This study was funded by Beijing municipal medical research institute public welfare development and reform pilot project (jingyiyan2019-6); The National Natural Science Foundation of China (81672026); the Chinese Foundation for Hepatitis Prevention and Control (TQGB20190050 and 2020033).
Author information
Authors and Affiliations
Contributions
YJ performed the experiments and analyzed the data. WL and YC enrolled the patients and provided specimens. PX participated in the collection of specimens. HS carried out routine maintenance and repair of the image flow cytometry. HS designed the study and wrote the paper. DC, YC, YM reviewed and edited the paper.
Corresponding authors
Ethics declarations
Conflict of interest
All authors (Yan Jiao, Wang Lu, Ping Xu, Honglin Shi, Dexi Chen, Yu Chen, Hongbo Shi, Yingmin Ma) have no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12072_2021_10217_MOESM1_ESM.tif
Supplementary file1 Supplement.1. The subgroup analysis of hepatocyte-derived exosome in different stages of patients with ACLF. (A) The image of exosomes with CD63 and ALB in different stages of patients with ACLF by image flow cytometry. (B) The percentage of exosomes with CD63 and ALB in different stages of patients with ACLF by image flow cytometry. (C) The comparison of exosomes with CD63 and ALB in different stages of patients with ACLF. (D) The image of exosomes with CD63 and ALB and VEGF in different stages of patients with ACLF by image flow cytometry. (E) The percentage of exosomes with CD63 and ALB and VEGF in different stages of patients with ACLF by image flow cytometry. (F) The comparison of exosomes with CD63 and ALB and VEGF in different stages of patients with ACLF. *indicated P<0.05, **indicated P<0.01. (TIF 19564 KB)
12072_2021_10217_MOESM2_ESM.tif
Supplementary file2 Supplement.2. The subgroup analysis of hepatocyte-derived exosome in different AFP levels of patients with ACLF. (A) The image of exosomes with CD63 and ALB in different AFP levels of patients with ACLF by image flow cytometry. (B) The percentage of exosomes with CD63 and ALB in different AFP levels of patients with ACLF by image flow cytometry. (C) The comparison of exosomes with CD63 and ALB in different AFP levels of patients with ACLF. (D) The image of exosomes with CD63 and ALB and VEGF in different AFP levels of patients with ACLF by image flow cytometry. (E) The percentage of exosomes with CD63 and ALB and VEGF in different AFP levels of patients with ACLF by image flow cytometry. (F) The comparison of exosomes with CD63 and ALB and VEGF in different AFP levels of patients with ACLF. *indicated P<0.05, **indicated P<0.01. (TIF 19573 KB)
Rights and permissions
About this article
Cite this article
Jiao, Y., Lu, W., Xu, P. et al. Hepatocyte-derived exosome may be as a biomarker of liver regeneration and prognostic valuation in patients with acute-on-chronic liver failure. Hepatol Int 15, 957–969 (2021). https://doi.org/10.1007/s12072-021-10217-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10217-3