Abstract
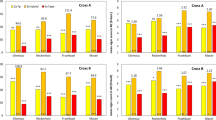
Species hybridization is key for the improvement of shrub willow (Salix spp.) bioenergy crops because hybrids often display heterosis for yield. The development of high-yielding genotypes requires numerous broad attempts at hybridization followed by field evaluation and selection for stable performance. Selection of improved shrub willow varieties for use as a bioenergy crop involves evaluation of full-sib progeny in family-based selection trials. Improving the accuracy of evaluation through the use of components of yield would greatly improve the efficiency of selection. Heterosis for biomass yield in intra- and interspecific F1 and F2 shrub willow crosses, made between Salix sections and ploidy, was examined by utilizing a suite of morphological, physiological, and chemical composition traits collected over the course of 12 weeks in the greenhouse and over 3 years in the field. Triploid families generated from diploid S. viminalis and tetraploid S. miyabeana displayed the greatest levels of heterosis for harvestable biomass and biomass-related growth traits in the greenhouse and in the field. While intraspecific S. purpurea diploids exhibited low levels of heterosis for these traits, interspecific diploids produced moderate levels of heterosis in greenhouse experiments. Differences between greenhouse and field trial results can largely be explained by pest damage, which negatively impacted interspecific diploids. Heterosis for the traits that form the basis for biomass yield, including stem growth, foliar, and physiological traits, was quantified, and family-level differences are discussed.






Similar content being viewed by others
References
Smart LB, Cameron KD (2012) Shrub Willow. In: Kole C, Joshi CP, Shonnard DR (eds) Handbook of bioenergy crop plants. CRC Press, Boca Raton, pp 687–708. https://doi.org/10.1201/b11711-32
Lauron-Moreau A, Pitre FE, Argus GW, Labrecque M, Brouillet L (2015) Phylogenetic relationships of American willows (Salix L., Salicaceae). PLoS ONE 10:e0121965. https://doi.org/10.1371/journal.pone.0121965
Argus GW (1997) Infrageneric classification of Salix (Salicaceae) in the New World. Syst Bot Monogr 52:1–121
Argus GW (1974) An experimental study of hybridization and pollination in Salix (willow). Can J Bot 52:1613–1619. https://doi.org/10.1139/b74-212
Tamura S, Kudo G (2000) Wind pollination and insect pollination of two temperate willow species, Salix miyabeana and Salix sachalinensis. Plant Ecol 147:185–192. https://doi.org/10.1023/A:1009870521175
Percy DM, Argus GW, Cronk QC, Fazekas AJ, Kesanakurti PR, Burgess KS, Husband BC, Newmaster SG, Barrett SCH, Graham SW (2014) Understanding the spectacular failure of DNA barcoding in willows (Salix): does this result from a trans-specific selective sweep? Mol Ecol 23:4737–4756. https://doi.org/10.1111/mec.12837
Hardig TM, Brunsfeld SJ, Fritz RS, Morgan M, Orians CM (2000) Morphological and molecular evidence for hybridization and introgression in a willow (Salix) hybrid zone. Mol Ecol 9:9–24. https://doi.org/10.1046/j.1365-294X.2000.00757.x
Lin J, Gibbs JP, Smart LB (2009) Population genetic structure of native versus naturalized sympatric shrub willows (Salix: Salicaceae). Am J Bot 96:771–785. https://doi.org/10.3732/ajb.0800321
Heribert-Nilsson N (1918) Experimentelle studien uber variabiliät, Spaltung, Artibildung, und evoluation in der gattung Salix. Lunds Universitats Arsskrift N. F. Avd 2
Karp A, Hanley S, Trybush S, Macalpine W, Pei MH, Shield I (2011) Genetic improvement of willow for bioenergy and biofuels. J Integ Plant Biol 53.https://doi.org/10.1111/j.1744-7909.2010.01015.x
Kuzovkina YA, Weih M, Romero MA, Charles J, Hust S, McIvor I, Karp A, Trybush S, Labrecque M, Teodorescu TI, Singh NB, Smart LB, Volk TA (2008) Salix: botany and global horticulture. Horticultural Reviews. Wiley, pp 447–489. https://doi.org/10.1002/9780470380147.ch8
Birchler JA, Yao H, Chudalayandi S (2006) Unraveling the genetic basis of hybrid vigor. Proc Natl Acad Sci U S A 103:12957–12958. https://doi.org/10.1073/pnas.0605627103
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, dePamphilis CW, Wall PK, Soltis PS (2009) Polyploidy and angiosperm diversification. Am J Bot 96:336–348. https://doi.org/10.3732/ajb.0800079
Wagner ND, He L, Hörandl E (2020) Phylogenomic relationships and evolution of polyploid Salix species revealed by RAD sequencing data. Front Plant Sci 11:1077. https://doi.org/10.3389/fpls.2020.01077
Chen ZJ (2010) Molecular mechanisms of polyploidy and hybrid vigor. Trends Plant Sci 15:57–71. https://doi.org/10.1016/j.tplants.2009.12.003
Volk TA, Abrahamson LP, Cameron KD, Castellano P, Corbin T, Fabio E, Johnson G, Kuzovkina-Eischen Y, Labrecque M, Miller R, Sidders D, Smart LB, Staver K, Stanosz GR, Kv R (2011) Yields of willow biomass crops across a range of sites in North America. Asp Appl Biol 112:67–74
Fabio ES, Volk TA, Miller RO, Serapiglia MJ, Kemanian AR, Montes F, Kuzovkina YA, Kling GJ, Smart LB (2017) Contributions of environment and genotype to variation in shrub willow biomass composition. Ind Crop Prod 108:149–161. https://doi.org/10.1016/j.indcrop.2017.06.030
Zsuffa L, Mosseler A, Raj Y (1984) Prospects for interspecific hybridization in willow for biomass production. In: Perttu KL (ed) Ecology and management of forest biomass production systems. Report 15: Swedish University of Agricultural Sciences, Uppsala
Orians CM (2000) The effects of hybridization in plants on secondary chemistry: implications for the ecology and evolution of plant–herbivore interactions. Am J Bot 87:1749–1756. https://doi.org/10.2307/2656824
Hallgren P, Ikonen A, Hjältén J, Roininen H (2003) Inheritance patterns of phenolics in F1, F2, and backcross hybrids of willows: implications for herbivore responses to hybrid plants. J Chem Ecol 29:1143–1158. https://doi.org/10.1023/A:1023829506473
Wang W, Carlson CH, Smart LB, Carlson JE (2020) Transcriptome analysis of contrasting resistance to herbivory by Empoasca fabae in two shrub willow species and their hybrid progeny. PLoS ONE 15:e0236586. https://doi.org/10.1371/journal.pone.0236586
Johansson LKH, Alstrom S (2000) Field resistance to willow leaf rust Melampsora epitea in inter- and intraspecific hybrids of Salix viminalis and S. dasyclados. Eur J Plant Pathol 106:763–769. https://doi.org/10.1023/a:1026573219481
Orians CM, Huang CH, Wild A, Dorfman KA, Zee P, Dao MTT, Fritz RS (1997) Willow hybridization differentially affects preference and performance of herbivorous beetles. Entomol Expr Appl 83:285–294. https://doi.org/10.1046/j.1570-7458.1997.00183.x
Orians CM, Griffiths ME, Roche BM, Fritz RS (2000) Phenolic glycosides and condensed tannins in Salix sericea, S. eriocephala and their F1 hybrids: not all hybrids are created equal. Biochem Syst Ecol 28:619–632. https://doi.org/10.1016/S0305-1978(99)00101-5
Orians CM, Fritz RS, Hochwender CG, Albrectsen BR, Czesak ME (2013) How slug herbivory of juvenile hybrid willows alters chemistry, growth and subsequent susceptibility to diverse plant enemies. Ann Bot 112:757–765. https://doi.org/10.1093/aob/mct002
Cameron KD, Phillips IS, Kopp RF, Volk TA, Maynard CA, Abrahamson LP, Smart LB (2008) Quantitative genetics of traits indicative of biomass production and heterosis in 34 full-sib F1 Salix eriocephala families. Bioenerg Res 1:80–90. https://doi.org/10.1007/s12155-008-9006-x
Fabio ES, Kemanian AR, Montes F, Miller RO, Smart LB (2017) A mixed model approach for evaluating yield improvements in interspecific hybrids of shrub willow, a dedicated bioenergy crop. Ind Crop Prod 96:57–70. https://doi.org/10.1016/j.indcrop.2016.11.019
Nilsson-Ehle H (1936) A gigasform found in nature from Populus tremula. Hereditas 21:379–382
Fabio ES, Volk TA, Miller RO, Serapiglia MJ, Gauch HG, Van Rees KC, Hangs RD, Amichev BY, Kuzovkina YA, Labrecque M (2016) Genotype by environment interactions analysis of North American shrub willow yield trials confirms superior performance of triploid hybrids. GCB Bioenergy 9:445–459. https://doi.org/10.1111/gcbb.12344
Serapiglia MJ, Gouker FE, Smart LB (2014) Early selection of novel triploid hybrids of shrub willow with improved biomass yield relative to diploids. BMC Plant Biol 14:74. https://doi.org/10.1186/1471-2229-14-74
Carlson CH, Gouker FE, Crowell CR, Evans L, DiFazio SP, Smart CD, Smart LB (2019) Joint linkage and association mapping of complex traits in shrub willow (Salix purpurea L.). Ann Bot 124:701–716. https://doi.org/10.1093/aob/mcz047
Carlson CH, Smart LB (2016) Electrical capacitance as a predictor of root dry weight in shrub willow (Salix; Salicaceae) parents and progeny. Appl Plant Sci 4:e1600031. https://doi.org/10.3732/apps.1600031
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67:48. https://doi.org/10.18637/jss.v067.i01
Velazco JG, Rodriguez-Alvarez MX, Boer MP, Jordan DR, Eilers PHC, Malosetti M, van Eeuwijk FA (2017) Modelling spatial trends in sorghum breeding field trials using a two-dimensional P-spline mixed model. Theor Appl Genet 130:1375–1392. https://doi.org/10.1007/s00122-017-2894-4
Rodríguez-Álvarez MX, Lee D-J, Kneib T, Durbán M, Eilers P (2015) Fast smoothing parameter separation in multidimensional generalized P-splines: the SAP algorithm. Stat Comp 25:941–957. https://doi.org/10.1007/s11222-014-9464-2
Faliński JB (1980) Vegetation dynamics and sex structure of the populations of pioneer dioecious woody plants. Vegetatio 43:23–38. https://doi.org/10.1007/BF00121014
Crawford R, Balfour J (1983) Female predominant sex ratios and physiological differentiation in arctic willows. J Ecol 71:149–160. https://doi.org/10.2307/2259968
Alliende M, Harper J (1989) Demographic studies of a dioecious tree. I. Colonization, sex and age structure of a population of Salix cinerea. J Ecol 77:1029–1047. https://doi.org/10.2307/2260821
Dawson TE, Bliss L (1989) Patterns of water use and the tissue water relations in the dioecious shrub, Salix arctica: the physiological basis for habitat partitioning between the sexes. Oecologia 79:332–343. https://doi.org/10.1007/BF00384312
Alström-Rapaport C, Lascoux M, Gullberg U (1997) Sex determination and sex ratio in the dioecious shrub Salix viminalis L. Theor Appl Genet 94:493–497. https://doi.org/10.1007/s001220050442
Ueno N, Suyama Y, Seiwa K (2007) What makes the sex ratio female-biased in the dioecious tree Salix sachalinensis? J Ecol 95:951–959. https://doi.org/10.1111/j.1365-2745.2007.01269.x
Che-Castaldo C, Crisafulli CM, Bishop JG, Fagan WF (2015) What causes female bias in the secondary sex ratios of the dioecious woody shrub Salix sitchensis colonizing a primary successional landscape? Am J Bot 102(8):1309–1322. https://doi.org/10.3732/ajb.1500143
Yin T, DiFazio SP, Gunter LE, Zhang X, Sewell MM, Woolbright SA, Allan GJ, Kelleher CT, Douglas CJ, Wang M, Tuskan GA (2008) Genome structure and emerging evidence of an incipient sex chromosome in Populus. Genome Res 18:422–430. https://doi.org/10.1101/gr.7076308
Gouker FE, Carlson CH, Zhu J, Evans LM, Smart CD, DiFazio SP, Smart LB (2021) Sexual dimorphism in the dioecious shrub willow, Salix purpurea L. Am J Bot (In press).https://doi.org/10.1101/2020.04.05.026427
Fabio ES, Smart LB (2018) Effects of nitrogen fertilization in shrub willow short rotation coppice production—a quantitative review. GCB Bioenergy 10:548–564. https://doi.org/10.1111/gcbb.12507
Acknowledgements
This research was supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research (grant no. DE-SC0008375) and the US Department of Agriculture National Institute for Food and Agriculture (grant no. 2018-68005-27925). We are grateful to Smart Lab members Curt Carter, Lauren Carlson, and Dawn Fishback for their excellent technical assistance and to Fred Gouker and Eric Fabio for assistance with harvesting and processing plants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carlson, C.H., Smart, L.B. Heterosis for Biomass-Related Traits in Interspecific Triploid Hybrids of Willow (Salix spp.). Bioenerg. Res. 15, 1042–1056 (2022). https://doi.org/10.1007/s12155-021-10305-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-021-10305-0