Abstract
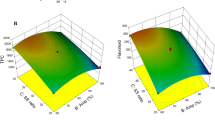
Citrus reticulata (kinnow mandarin) peels, which contain a plethora of bioactive compounds having nutraceutical properties, are discarded as waste, by the citrus processing industries. This organic waste holds a tremendous potential to be converted into nutritious value-added products, using various extraction approaches. Extraction helps in isolation of bioactive phytochemicals from the by-products that could be used as nutritional supplements in food systems. This study was carried out to standardize process parameters of probe sonicator, using response surface methodology, for separation of bioactives from kinnow mandarin peels. The crude extract obtained was analyzed for total phenolic content as well as antioxidant activity, using spectrophotometric analysis. Maximum TPC (36.17 mg GAE/g extract), DPPH radical scavenging activity (64.70% DPPH reduction), ferric reducing antioxidant power assay (28.17 mM/100 g), superoxide radical scavenging activity (57.37%) and hydroxyl radical scavenging activity (60.30%), were observed at an amplitude of 31%, liquid to solid ratio of 30:1 at 41 °C temperature after treatment time of 15 min. The extract obtained at optimized conditions was also analyzed via Fourier Transform Infrared Spectroscopy (FTIR) to validate various functional groups observed in the extract. This study can be helpful for developing a scale-up process, for utilization of nutraceutical potential of kinnow peels and development of functional foods. Thus, biorefining of kinnow mandarin peels can be beneficial for waste management in citrus industry.



Similar content being viewed by others
References
Saini A, Panesar PS, Bera MB (2020) Valuation of Citrus reticulata (kinnow) peels for the extraction of lutein using ultrasonication technique. Biomass Convers Biorefin: 1–9. https://doi.org/10.1007/s13399-020-00605-4
FAO (2019) FAOSTAT [WWW Document]. Food Agric. Organ. United Nations. http://www.fao.org/faostat/en/#data/QC. (Accessed 25 Feb 2021)
Ait‐Oubahou A, Benichou M, Sagar M, Kaanane A, Yahia EM (2017) Citrus. In: Yahia EM (2nd) Fruit and Vegetable Phytochemicals: Chemistry and Human Health, Wiley Blackwell, US, pp 1003–1022
Castillo-Herrera GA, Farías-Álvarez LJ, García-Fajardo JA, Delgado-Saucedo JI, Puebla-Pérez AM, Lugo-Cervantes E (2015) Bioactive extracts of Citrus aurantifolia swingle seeds obtained by supercritical CO2 and organic solvents comparing its cytotoxic activity against L5178Y leukemia lymphoblasts. J Supercrit Fluid 101:81–86. https://doi.org/10.1016/j.supflu.2015.02.026
Singh B, Singh JP, Kaur A, Singh N (2020) Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res Int 132:109114. https://doi.org/10.1016/j.foodres.2020.109114
Sagar NA, Pareek S, Sharma S, Yahia EM, Lobo MG (2018) Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci F 17(3):512–531. https://doi.org/10.1111/1541-4337.12330
Espiard E (2002) Introduction à la transformation industrielle des fruits. Éd. Tec & Doc
Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI (2013) Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem 24(8):1415–1422. https://doi.org/10.1016/j.jnutbio.2013.05.001
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci IJBS 4(2):89
Ma YQ, Chen JC, Liu DH, Ye XQ (2009) Simultaneous extraction of phenolic compounds of citrus peels extracts: effect of ultrasound. Ultrasonsonochem 16(1):57–62. https://doi.org/10.1016/j.ultsonch.2008.04.012
Dilas S, Canadanović-Brunet J, Cetkovic G (2009) By-products of fruits processing as a source of phytochemicals. Chem Ind Chem Eng Q./CICEQ 15(4):191–202. https://doi.org/10.2298/CICEQ0904191D
Rezzadori K, Benedetti S, Amante ER (2012) Proposals for the residue’s recovery: orange waste as raw material for new products. Food Bioprod Process 90(4):606–614. https://doi.org/10.1016/j.fbp.2012.06.002
Montero-Calderon A, Cortes C, Zulueta A, Frigola A, Esteve MJ (2019) Green solvents and ultrasound-assisted extraction of bioactive orange (Citrus sinensis) peels compounds. Sci Rep 9(1):1–8. https://doi.org/10.1038/s41598-019-52717-1
Nipornram S, Tochampa W, Rattanatraiwong P, Singanusong R (2018) Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peels. Food Chem 241:338–345. https://doi.org/10.1016/j.foodchem.2017.08.114
Nishad J, Saha S, Kaur C (2019) Enzyme‐and ultrasound‐assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: a comparative study. J Food Process Preserv 43(8):e14046. https://doi.org/10.1111/jfpp.14046
Hosseini SS, Khodaiyan F, Kazemi M, Najari Z (2019) Optimization and characterization of pectin extracted from sour orange peels by ultrasound assisted method. Int J Biol Macromol 125:621–629. https://doi.org/10.1016/j.ijbiomac.2018.12.096
Omar J, Alonso I, Garaikoetxea A, Etxebarria N (2013) Optimization of focused ultrasound extraction (FUSE) and supercritical fluid extraction (SFE) of citrus peels volatile oils and antioxidants. Food Anal Methods 6(4):1244–1252. https://doi.org/10.1007/s12161-012-9536-x
Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, Saddozai AA (2017) Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal 25(3):488–500. https://doi.org/10.1016/j.jfda.2016.07.010
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/S0076-6879(99)99017-1
Brand-Williams W, Cuvelier ME, Berset CLWT (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Azman NFIN, Azlan A, Khoo HE, Razman MR (2019) Antioxidant properties of fresh and frozen peels of citrus species. Curr Res Nutr Food Sci J 7(2):331–339
Shehata MG, Awad TS, Asker DH, Abd El-Aziz NM, Youssef MM (2021) Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr Res FoodSci 4:326–335. https://doi.org/10.1016/j.crfs.2021.05.001
Rangarajan N, Sampath V, Prakash D (2018) Spectral studies and biochemical evaluation of citrus limon Pulp. Int J Pharmacogn Phytochem Res 10(06):246–253
Liew SS, Ho WY, Yeap SK, Sharifudin SAB (2018) Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peels extracts. Peer J 6:e5331. https://doi.org/10.7717/peerj.5331
Zhang H, Yang YF, Zhou ZQ (2018) Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J Integr Agric 17(1):256–263
Gil-Chavez GJ, Villa JA, Ayala-Zavala JF, Basilio Heredia J, Sepulveda D, Yahia EM (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr Rev Food Sci Food Saf 12:5–23
Chan SW, Lee CY, Yap CF, Wan Aida WM, Ho CW (2009) Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int Food Res J 16(2):203–213
Papoutsis K, Pristijono P, Golding JB, Stathopoulos CE, Bowyer MC, Scarlett CJ, Vuong QV (2016) Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int J Food Sci Technol 51(9):2009–2018
Saha J, Biswas A, Chhetri A, Sarkar PK (2011) Response surface optimization of antioxidant extraction from kinema, a Bacillus-fermented soybean food. Food Chem 129(2):507–513. https://doi.org/10.1016/j.foodchem.2011.04.108
Xu G, Ye X, Chen J, Liu D (2007) Effect of heat treatment on the phenolic compounds and antioxidant capacity of citrus peel extract. J Agric Food Chem 55(2):330–335
Iglesias-Carres L, Mas-Capdevila A, Bravo FI, Aragonès G, Muguerza B, Arola-Arnal A (2019) Optimization of a polyphenol extraction method for sweet orange pulp (Citrus sinensis L.) to identify phenolic compounds consumed from sweet oranges. PLoS One 14(1):e0211267. https://doi.org/10.1371/journal.pone.0211267
Cacace JE, Mazza G (2003) Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng 59(4):379–389. https://doi.org/10.1016/s0260-8774(02)00497-1
Stanisavljević IT, Veličković DT, Todorović ZB, Lazić ML, Veljković VB (2009) Comparison of techniques for the extraction of tobacco seed oil. Eur J Lipid Sci Technol 111:513–518. https://doi.org/10.1002/ejlt.200800232
Jerman T, Trebše P, Vodopivec BM (2010) Ultrasound-assisted solid-liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem 123:175–182. https://doi.org/10.1016/j.foodchem.2010.04.006
Ma YQ, Chen JC, Liu DH, Ye XQ (2008) Effect of ultrasonic treatment on the total phenolic and antioxidant activity of extracts from citrus peel. J Food Sci 73(8):T115–T120
Sharayei P, Azarpazhooh E, Zomorodi S, Ramaswamy HS (2019) Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peels. LWT 101:342–350. https://doi.org/10.1016/j.foodchem.2010.04.006
Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC (2004) Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J Agric Food Chem 52(11):3389–3393
Zulkifli KS, Abdullah N, Abdullah A, Aziman N, Kamarudin WSSW (2012) Bioactive phenolic compounds and antioxidant activity of selected fruit peels. In International Conference on Environment, Chemistry and Biology (Vol. 49, No. 14, pp. 66–70)
Tumbas VT, Ćetković GS, Đilas SM, Čanadanović-Brunet JM, Vulić JJ, Knez Ž, Škerget M (2010) Antioxidant activity of mandarin (Citrus reticulata) peel. Acta Period Technol 41:195–203
Dobiáš P, Pavlíková P, Adam M, Eisner A, Beňová B, Ventura K (2010) Comparison of pressurised fluid and ultrasonic extraction methods for analysis of plant antioxidants and their antioxidant capacity. Cent Eur J Chem 8(1):87–95
Yuan CG, Huo C, Gui B, Cao WP (2016) Green synthesis of gold nanoparticles using Citrus maxima peel extract and their catalytic/antibacterial activities. IET Nanobiotechnol 11(5):523–530. https://doi.org/10.1049/iet-nbt.2016.0183
Acknowledgements
The authors would like to acknowledge the financial support provided by Council of Scientific and Industrial Research (CSIR) under research scheme 38(1491)/19/EMR-II. Further, the infrastructural support provided by Sant Longowal Institute of Engineering and Technology (SLIET), Longowal, Punjab, India, for carrying out the research is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, S., Panesar, P.S. & Chopra, H.K. Standardization of ultrasound-assisted extraction of bioactive compounds from kinnow mandarin peel. Biomass Conv. Bioref. 13, 8853–8863 (2023). https://doi.org/10.1007/s13399-021-01674-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01674-9