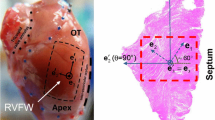
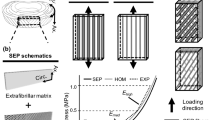
Abstract
The mechanics of most soft tissues in the human body are determined by the organization of their collagen fibers. Predicting how mechanics will change during growth and remodeling of those tissues requires constitutive laws that account for the density and dispersion of collagen fibers. Post-infarction scar in the heart, a mechanically and structurally complex material, does not yet have a validated fiber-based constitutive model. In this study, we tested four different constitutive laws employing exponential or polynomial strain-energy functions and accounting for either mean fiber orientation alone or the details of the fiber distribution about that mean. We quantified the goodness of fit of each law to mechanical testing data from 6-week-old myocardial scar in the rat using both sum of squared error (SSE) and the Akaike Information Criterion (AIC) to account for differences in the number of material parameters within the constitutive laws. We then compared their ability to prospectively predict the mechanics of independent myocardial scar samples from other time points during healing. Our analysis suggests that a constitutive law with a polynomial form that incorporates detailed information about collagen fiber distribution using a structure tensor provides excellent fits with just two parameters and reasonable predictions of myocardial scar mechanics from measured structure alone in scars containing sufficiently high collagen content.








Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Holmes, J.W., Nuñez, J.A., Covell, J.W.: Functional implications of myocardial scar structure. Am. J. Physiol., Heart Circ. Physiol. 272, 2123–2130 (1997). https://doi.org/10.1152/ajpheart.1997.272.5.h2123
Fomovsky, G.M., Holmes, J.W.: Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am. J. Physiol., Heart Circ. Physiol. 298, 221–228 (2010). https://doi.org/10.1152/ajpheart.00495.2009
Richardson, W., Clarke, S.A., Quinn, T., Holmes, J.W.: Physiological implications of myocardial scar structure. Compr. Physiol. 5, 1877–1909 (2016). https://doi.org/10.1002/cphy.c140067.Physiological
Richardson, W.J., Holmes, J.W.: Why is infarct expansion such an elusive therapeutic target? J. Cardiovasc. Transl. Res. 8, 421–430 (2015). https://doi.org/10.1007/s12265-015-9652-2
Weir, R.A.P., McMurray, J.J.V., Velazquez, E.J.: Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am. J. Cardiol. 97, 13–25 (2006). https://doi.org/10.1016/j.amjcard.2006.03.005
Rouillard, A.D., Holmes, J.W.: Coupled agent-based and finite-element models for predicting scar structure following myocardial infarction. Prog. Biophys. Mol. Biol. 115, 235–243 (2014). https://doi.org/10.1016/j.pbiomolbio.2014.06.010
Fomovsky, G.M., Rouillard, A.D., Holmes, J.W.: Regional mechanics determine collagen fiber structure in healing myocardial infarcts. J. Mol. Cell. Cardiol. 52, 1083–1090 (2012). https://doi.org/10.1016/j.yjmcc.2012.02.012
Caggiano, L.R., Lee, J., Holmes, J.W.: Surgical reinforcement alters collagen alignment and turnover in healing myocardial infarcts. Am. J. Physiol. Circ. Physiol. 315, 1041–1050 (2018). https://doi.org/10.1152/ajpheart.00088.2018
Guccione, J., Mcculloch, A., Waldman, L.: Passive material properties of intact ventricular myocardium determined from a cylindrical model. J. Biomech. Eng. 118, 262 (1996). https://doi.org/10.1115/1.2795971
Costa, K.D., Holmes, J.W., McCulloch, A.D.: Modelling cardiac mechanical properties. Philos. Trans. A. Math. Phys. Eng. Sci. 359, 1233–1250 (2001)
Humphrey, J., Strumpf, R., Yin, F.: Determination of a constitutive relation for passive myocardium: I. A new functional form. J. Biomech. Eng. 112, 333–339 (1990)
Holzapfel, G.A., Ogden, R.W.: Constitutive modelling of passive myocardium: a structurally based framework for material characterization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 367, 3445–3475 (2009). https://doi.org/10.1098/rsta.2009.0091
Billiar, K.L., Sacks, M.S.: Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II—A structural constitutive model. J. Biomech. Eng. 122, 327–335 (2000). https://doi.org/10.1115/1.1287158
Guccione, J.M., Moonly, S.M., Moustakidis, P., Costa, K.D., Moulton, M.J., Ratcliffe, M.B., Pasque, M.K.: Mechanism underlying mechanical dysfunction in the border zone of left ventricular aneurysm: a finite element model study. Ann. Thorac. Surg. 71, 654–662 (2001). https://doi.org/10.1016/S0003-4975(00)02338-9
Dang, A.B.C., Guccione, J.M., Zhang, P., Wallace, A.W., Gorman, R.C., Gorman, J.H., Ratcliffe, M.B.: Effect of ventricular size and patch stiffness in surgical anterior ventricular restoration: a finite element model study. Ann. Thorac. Surg. 79, 185–193 (2005). https://doi.org/10.1016/j.athoracsur.2004.06.007
Sun, K., Stander, N., Jhun, C.S., Zhang, Z., Suzuki, T., Wang, G.Y., Saeed, M., Wallace, A.W., Tseng, E.E., Baker, A.J., Saloner, D., Einstein, D.R., Ratcliffe, M.B., Guccione, J.M.: A computationally efficient formal optimization of regional myocardial contractility in a sheep with left ventricular aneurysm. J. Biomech. Eng. 131, 1–10 (2009). https://doi.org/10.1115/1.3148464
Walker, J.C., Ratcliffe, M.B., Zhang, P., Wallace, A.W., Fata, B., Hsu, E.W., Saloner, D., Guccione, J.M.: MRI-based finite-element analysis of left ventricular aneurysm. Am. J. Physiol., Heart Circ. Physiol. 289, 692–700 (2005). https://doi.org/10.1152/ajpheart.01226.2004
Wall, S.T., Walker, J.C., Healy, K.E., Ratcliffe, M.B., Guccione, J.M.: Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation 114, 2627–2635 (2006). https://doi.org/10.1161/CIRCULATIONAHA.106.657270
Gupta, K.B., Ratcliffe, M.B., Fallert, M.A., Edmunds, L.H., Bogen, D.K.: Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation 89, 2315–2326 (1994). https://doi.org/10.1161/01.CIR.89.5.2315
Sirry, M.S., Butler, J.R., Patnaik, S.S., Brazile, B., Bertucci, R., Claude, A., McLaughlin, R., Davies, N.H., Liao, J., Franz, T.: Infarcted rat myocardium: data from biaxial tensile and uniaxial compressive testing and analysis of collagen fibre orientation. Data Br. 8, 1338–1343 (2016). https://doi.org/10.1016/j.dib.2016.08.005
Brazile, B.L., Butler, J.R., Patnaik, S.S., Claude, A., Prabhu, R., Williams, L.N., Perez, K.L., Nguyen, K.T., Zhang, G., Bajona, P., Peltz, M., Yang, Y., Hong, Y., Liao, J.: Biomechanical properties of acellular scar ECM during the acute to chronic stages of myocardial infarction. J. Mech. Behav. Biomed. Mater. 116, 104342 (2021). https://doi.org/10.1016/j.jmbbm.2021.104342
Whittaker, P., Kloner, R.A., Boughner, D.R., Pickering, J.G.: Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 89, 397–410 (1994). https://doi.org/10.1007/BF00788278
Gasser, T.C., Ogden, R.W., Holzapfel, G.A.: Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3, 15–35 (2006). https://doi.org/10.1098/rsif.2005.0073
Schmid, H., Nash, M.P., Young, A.A., Hunter, P.J.: Myocardial material parameter estimation - a comparative study for simple shear. J. Biomech. Eng. 128, 742–750 (2006). https://doi.org/10.1115/1.2244576
Akaike, H.: Information theory and an extension of the maximum likelihood principle. In: Parzen, E., Tanabe, K., Kitagawa, G. (eds.) Selected Papers of Hirotugu Akaike, pp. 267–281. Springer, New York (1973)
Akaike, H.: A new look at the statistical model identification. IEEE Trans. Autom. Control 19, 716–723 (1974). https://doi.org/10.1109/TAC.1974.1100705
Holmes, J.W., Borg, T.K., Covell, J.W.: Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 7, 223–253 (2005). https://doi.org/10.1146/annurev.bioeng.7.060804.100453
Fomovsky, G.M., Clark, S.A., Parker, K.M., Ailawadi, G., Holmes, J.W.: Anisotropic reinforcement of acute anteroapical infarcts improves pump function. Circ. Heart Fail. 5, 515–522 (2012). https://doi.org/10.1161/CIRCHEARTFAILURE.111.965731
Holmes, J.W., Laksman, Z., Gepstein, L.: Making better scar: emerging approaches for modifying mechanical and electrical properties following infarction and ablation. Prog. Biophys. Mol. Biol. 120, 134–148 (2016). https://doi.org/10.1016/j.pbiomolbio.2015.11.002
Clarke, S.A., Richardson, W.J., Holmes, J.W.: Modifying the mechanics of healing infarcts: is better the enemy of good? J. Mol. Cell. Cardiol. 93, 115–124 (2016). https://doi.org/10.1016/j.yjmcc.2015.11.028
Sacks, M.S.: Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 125, 280–287 (2003). https://doi.org/10.1115/1.1544508
Acknowledgements
This work was supported by National Institutes of Health grants R01 HL-116449 and R01 EB-137755.
Funding
This study was funded by NIH R01 HL-116449 and R01 EB-137755.
Author information
Authors and Affiliations
Contributions
Study design, data collection, and analysis were performed by Laura R. Caggiano and Jeffrey W. Holmes. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experiments were approved by the University of Virginia Institutional Animal Care and Use Committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Caggiano, L.R., Holmes, J.W. A Comparison of Fiber Based Material Laws for Myocardial Scar. J Elast 145, 321–337 (2021). https://doi.org/10.1007/s10659-021-09845-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10659-021-09845-5