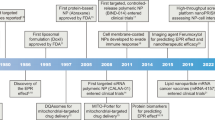
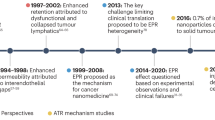
Abstract
Cancer nanomedicines were initially envisioned as magic bullets, travelling through the circulation to target tumours while sparing healthy tissues the toxicity of classic chemotherapy. While a limited number of nanomedicine therapies have resulted, the disappointing news is that major obstacles were overlooked in the nanoparticle’s journey. However, some of these challenges may be turned into opportunities. Here, we discuss biological barriers to cancer nanomedicines and elaborate on two directions that the field is currently exploring to meet its initial expectations. The first strategy entails re-engineering cancer nanomedicines to prevent undesired interactions en route to the tumour. The second aims instead to leverage these obstacles into out-of-the-box diagnostic and therapeutic applications of nanomedicines, for cancer and beyond. Both paths require, among other developments, a deeper understanding of nano–bio interactions. We offer a forward look at how classic cancer nanomedicine may overcome its limitations while contributing to other areas of research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nichols, J. W. & Bae, Y. H. EPR: evidence and fallacy. J. Control. Release 190, 451–464 (2014).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Gerlowski, L. E. & Jain, R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 31, 288–305 (1986).
Landgraf, M. et al. Targeted camptothecin delivery via silicon nanoparticles reduces breast cancer metastasis. Biomaterials 240, 119791 (2020).
Chen, C. et al. Reversibly-regulated drug release using poly(tannic acid) fabricated nanocarriers for reduced secondary side effects in tumor therapy. Nanoscale Horiz. 5, 986–998 (2020).
Al-Ahmady, Z. S., Chaloin, O. & Kostarelos, K. Monoclonal antibody-targeted, temperature-sensitive liposomes: in vivo tumor chemotherapeutics in combination with mild hyperthermia. J. Control. Release 196, 332–343 (2014).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010).
Quintanilla, M. et al. Thermal monitoring during photothermia: hybrid probes for simultaneous plasmonic heating and near-infrared optical nanothermometry. Theranostics 9, 7298–7312 (2019).
Feng, L., Gai, S., He, F., Yang, P. & Zhao, Y. Multifunctional bismuth ferrite nanocatalysts with optical and magnetic functions for ultrasound-enhanced tumor theranostics. ACS Nano 14, 7245–7258 (2020).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, e10143 (2019).
He, H., Liu, L., Morin, E. E., Liu, M. & Schwendeman, A. Survey of clinical translation of cancer nanomedicines—lessons learned from successes and failures. Acc. Chem. Res. 52, 2445–2461 (2019).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Sofias, A. M. et al. Tumor targeting by αvβ3-integrin-specific lipid nanoparticles occurs via phagocyte hitchhiking. ACS Nano 14, 7832–7846 (2020).
Salvati, A. et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 8, 137–143 (2013).
Lazarovits, J., Chen, Y. Y., Sykes, E. A. & Chan, W. C. Nanoparticle–blood interactions: the implications on solid tumour targeting. Chem. Commun. 51, 2756–2767 (2015).
Chen, F. et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat. Nanotechnol. 12, 387–393 (2017).
Ju, Y. et al. Person-specific biomolecular coronas modulate nanoparticle interactions with immune cells in human blood. ACS Nano 14, 15723–15737 (2020).
Shah, N. B. et al. Blood–nanoparticle interactions and in vivo biodistribution: impact of surface PEG and ligand properties. Mol. Pharm. 9, 2146–2155 (2012).
Campbell, F. et al. Directing nanoparticle biodistribution through evasion and exploitation of stab2-dependent nanoparticle uptake. ACS Nano 12, 2138–2150 (2018).
Hayashi, Y. et al. Differential nanoparticle sequestration by macrophages and scavenger endothelial cells visualized in vivo in real-time and at ultrastructural resolution. ACS Nano 14, 1665–1681 (2020).
Balasubramanian, S. K. et al. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials 31, 2034–2042 (2010).
Miao, L. & Huang, L. Exploring the tumor microenvironment with nanoparticles. Cancer Treat. Res. 166, 193–226 (2015).
Hansen, A. E. et al. Positron emission tomography based elucidation of the enhanced permeability and retention effect in dogs with cancer using copper-64 liposomes. ACS Nano 9, 6985–6995 (2015).
de Lazaro, I. & Mooney, D. J. A nanoparticle’s pathway into tumours. Nat. Mater. 19, 486–487 (2020).
Weniger, M., Honselmann, K. C. & Liss, A. S. The extracellular matrix and pancreatic cancer: a complex relationship. Cancers 10, 316 (2018).
Lee, H., Fonge, H., Hoang, B., Reilly, R. M. & Allen, C. The effects of particle size and molecular targeting on the intratumoral and subcellular distribution of polymeric nanoparticles. Mol. Pharm. 7, 1195–1208 (2010).
Chauhan, V. P. et al. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew. Chem. Int. Ed. 50, 11417–11420 (2011).
Stylianopoulos, T. et al. Diffusion of particles in the extracellular matrix: the effect of repulsive electrostatic interactions. Biophys. J. 99, 1342–1349 (2010).
Miller, M. A. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(iv) pro-drug. Nat. Commun. 6, 8692 (2015).
Korangath, P. et al. Nanoparticle interactions with immune cells dominate tumor retention and induce T cell-mediated tumor suppression in models of breast cancer. Sci. Adv. 6, eaay1601 (2020).
Donahue, N. D., Acar, H. & Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 143, 68–96 (2019).
de Lazaro, I. et al. Graphene oxide as a 2D platform for complexation and intracellular delivery of siRNA. Nanoscale 11, 13863–13877 (2019).
Price, L. S. L., Stern, S. T., Deal, A. M., Kabanov, A. V. & Zamboni, W. C. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci. Adv. 6, eaay9249 (2020).
Park, K. The beginning of the end of the nanomedicine hype. J. Control. Release 305, 221–222 (2019).
Huang, S. K. et al. Extravasation and transcytosis of liposomes in Kaposi’s sarcoma-like dermal lesions of transgenic mice bearing the HIV tat gene. Am. J. Pathol. 143, 10–14 (1993).
Liu, X., Jiang, J. & Meng, H. Transcytosis—an effective targeting strategy that is complementary to ‘EPR effect’ for pancreatic cancer nano drug delivery. Theranostics 9, 8018–8025 (2019).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Liu, X. et al. Tumor-penetrating peptide enhances transcytosis of silicasome-based chemotherapy for pancreatic cancer. J. Clin. Invest. 127, 2007–2018 (2017).
Huo, D., Jiang, X. & Hu, Y. Recent advances in nanostrategies capable of overcoming biological barriers for tumor management. Adv. Mater. 32, e1904337 (2020).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Jin, Q., Deng, Y., Chen, X. & Ji, J. Rational design of cancer nanomedicine for simultaneous stealth surface and enhanced cellular uptake. ACS Nano 13, 954–977 (2019).
Hama, S. et al. Overcoming the polyethylene glycol dilemma via pathological environment-sensitive change of the surface property of nanoparticles for cellular entry. J. Control. Release 206, 67–74 (2015).
Hatakeyama, H. et al. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 14, 68–77 (2007).
Ouyang, B. et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 19, 1362–1371 (2020).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Meng, H. et al. Two-wave nanotherapy to target the stroma and optimize gemcitabine delivery to a human pancreatic cancer model in mice. ACS Nano 7, 10048–10065 (2013).
Seki, T. et al. Tumour necrosis factor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway. J. Control. Release 156, 381–389 (2011).
Bai, X. et al. Toward a systematic exploration of nano–bio interactions. Toxicol. Appl. Pharmacol. 323, 66–73 (2017).
Walkey, C. D. et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano 8, 2439–2455 (2014).
Lazarovits, J. et al. Supervised learning and mass spectrometry predicts the in vivo fate of nanomaterials. ACS Nano 13, 8023–8034 (2019).
Yaari, Z. et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 7, 13325 (2016).
Paunovska, K. et al. A direct comparison of in vitro and in vivo nucleic acid delivery mediated by hundreds of nanoparticles reveals a weak correlation. Nano Lett. 18, 2148–2157 (2018).
Jiang, W. et al. Designing nanomedicine for immuno-oncology. Nat. Biomed. Eng. 1, 0029 (2017).
Hadjidemetriou, M. et al. The human in vivo biomolecule corona onto pegylated liposomes: a proof-of-concept clinical study. Adv. Mater. 31, e1803335 (2019).
Papafilippou, L., Claxton, A., Dark, P., Kostarelos, K. & Hadjidemetriou, M. Protein corona fingerprinting to differentiate sepsis from non-infectious systemic inflammation. Nanoscale 12, 10240–10253 (2020).
Blume, J. E. et al. Rapid, deep and precise profiling of the plasma proteome with multi-nanoparticle protein corona. Nat. Commun. 11, 3662 (2020).
Corbo, C. et al. Analysis of the human plasma proteome using multi-nanoparticle protein corona for detection of Alzheimer’s disease. Adv. Health. Mater. 10, e2000948 (2021).
Ren, J. et al. Precision nanomedicine development based on specific opsonization of human cancer patient-personalized protein coronas. Nano Lett. 19, 4692–4701 (2019).
Min, Y. et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 12, 877–882 (2017).
Wang, M. et al. NIR-triggered phototherapy and immunotherapy via an antigen-capturing nanoplatform for metastatic cancer treatment. Adv. Sci. 6, 1802157 (2019).
Lazarovits, J. et al. Synthesis of patient-specific nanomaterials. Nano Lett. 19, 116–123 (2019).
Smith, B. R. et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat. Nanotechnol. 9, 481–487 (2014).
Chu, D., Dong, X., Zhao, Q., Gu, J. & Wang, Z. Photosensitization priming of tumor microenvironments improves delivery of nanotherapeutics via neutrophil infiltration. Adv. Mater. 29, 1701021 (2017).
Chu, D., Gao, J. & Wang, Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano 9, 11800–11811 (2015).
Irvine, D. J. & Dane, E. L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 20, 321–334 (2020).
Getts, D. R. et al. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat. Biotechnol. 30, 1217–1224 (2012).
Liu, Q. et al. Use of polymeric nanoparticle platform targeting the liver to induce Treg-mediated antigen-specific immune tolerance in a pulmonary allergen sensitization model. ACS Nano 13, 4778–4794 (2019).
Yeste, A., Nadeau, M., Burns, E. J., Weiner, H. L. & Quintana, F. J. Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 109, 11270–11275 (2012).
Rodell, C. B. et al. Tlr7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2, 578–588 (2018).
Qian, Y. et al. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano 11, 9536–9549 (2017).
de Lázaro, I. et al. Deep tissue translocation of graphene oxide sheets in human glioblastoma 3D spheroids and an orthotopic xenograft model. Adv. Ther. 4, 2000109 (2021).
Swierczewska, M., Kozlov, S. & Adiseshaiah, P. P. in Oncogenomics (eds Dammacco, F. & Silvestris, F.) 313–327 (Academic, 2019).
Landgraf, M., McGovern, J. A., Friedl, P. & Hutmacher, D. W. Rational design of mouse models for cancer research. Trends Biotechnol. 36, 242–251 (2018).
Carvalho, M. R. et al. Colorectal tumor-on-a-chip system: a 3D tool for precision onco-nanomedicine. Sci. Adv. 5, eaaw1317 (2019).
Albanese, A., Lam, A. K., Sykes, E. A., Rocheleau, J. V. & Chan, W. C. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat. Commun. 4, 2718 (2013).
Wang, H. F. et al. Tumor-vasculature-on-a-chip for investigating nanoparticle extravasation and tumor accumulation. ACS Nano 12, 11600–11609 (2018).
Chen, Y. Y., Syed, A. M., MacMillan, P., Rocheleau, J. V. & Chan, W. C. W. Flow rate affects nanoparticle uptake into endothelial cells. Adv. Mater. 32, e1906274 (2020).
Faria, M. et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 13, 777–785 (2018).
Leong, H. S. et al. On the issue of transparency and reproducibility in nanomedicine. Nat. Nanotechnol. 14, 629–635 (2019).
Bustin, S. & Nolan, T. Talking the talk, but not walking the walk: RT-qPCR as a paradigm for the lack of reproducibility in molecular research. Eur. J. Clin. Invest. 47, 756–774 (2017).
van der Meel, R. et al. Smart cancer nanomedicine. Nat. Nanotechnol. 14, 1007–1017 (2019).
Judson, I. et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL®/CAELYX®) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur. J. Cancer 37, 870–877 (2001).
Rifkin, R. M., Gregory, S. A., Mohrbacher, A. & Hussein, M. A. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma. Cancer 106, 848–858 (2006).
Xing, M., Yan, F., Yu, S. & Shen, P. Efficacy and cardiotoxicity of liposomal doxorubicin-based chemotherapy in advanced breast cancer: a meta-analysis of ten randomized controlled trials. PLoS ONE 10, e0133569–e0133569 (2015).
Xu, X. et al. Clinical comparison between paclitaxel liposome (Lipusu®) and paclitaxel for treatment of patients with metastatic gastric cancer. Asian Pac. J. Cancer Prev. 14, 2591–2594 (2013).
Tossey, J. C. et al. Comparison of conventional versus liposomal irinotecan in combination with fluorouracil for advanced pancreatic cancer: a single-institution experience. Med. Oncol. 36, 87 (2019).
Kang, Y. K. et al. Efficacy and safety findings from DREAM: a phase III study of DHP107 (oral paclitaxel) versus i.v. paclitaxel in patients with advanced gastric cancer after failure of first-line chemotherapy. Ann. Oncol. 29, 1220–1226 (2018).
Van Norman, G. A. Drugs, devices, and the FDA: Part 1: An overview of approval processes for drugs. JACC Basic Transl. Sci. 1, 170–179 (2016).
Acknowledgements
We acknowledge funding from the National Cancer Institute of the National Institutes of Health under award numbers U01CA214369 and U54CA244726. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We apologize to colleagues whose relevant publications were not cited due to space limitations.
Author information
Authors and Affiliations
Contributions
I.d.L. and D.J.M. conceived and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks Shuming Nie and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Lázaro, I., Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 20, 1469–1479 (2021). https://doi.org/10.1038/s41563-021-01047-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01047-7
This article is cited by
-
Microneedle-mediated nanomedicine to enhance therapeutic and diagnostic efficacy
Nano Convergence (2024)
-
Physiological principles underlying the kidney targeting of renal nanomedicines
Nature Reviews Nephrology (2024)
-
Redox-responsive polymer micelles co-encapsulating immune checkpoint inhibitors and chemotherapeutic agents for glioblastoma therapy
Nature Communications (2024)
-
Thiol-mediated transportation pathway: an approach for improving tumor penetration of nanomedicines in vivo
Science China Chemistry (2024)
-
Synergistic effect of mesoporous silica nanocarrier-assisted photodynamic therapy and anticancer agent activity on lung cancer cells
Lasers in Medical Science (2024)