Abstract
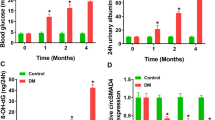
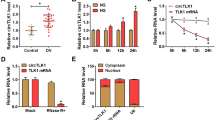
Diabetic nephropathy (DN) seriously affects the people's life and health in China. This study aimed to investigate the effect of circRNA circ-ITCH on improving DN by regulating the miR-33a-5p/SIRT6 axis and the possible mechanism of action. High glucose (HG)-induced rat mesangial cells (RMCs) were used to simulate the DN in vitro. Reverse transcription-quantitative PCR (RT-qPCR) and western blot analysis were conducted to detect the gene or protein expression. Cell Counting Kit-8 (CCK-8) and wound healing assays were performed to estimate the cell viability and migration capability. Immunofluorescence and enzyme-linked immunosorbent assay (ELISA) were used to detect the α-Smooth Muscle Actin (α-SMA) expression and levels of inflammatory factors. The potential associations between circ-ITCH and miR-33a-5p, miR-33a-5p and SIRT6 in RMCs were measured via dual-luciferase reporter assay. Streptozotocin (STZ) was used to induce the diabetic mice. Blood glucose and serum insulin of mice were determined by corresponding kits, and blood urea nitrogen (BUN) and serum creatinine (SCr) were measured using an automatic biochemical analyzer. Hematoxylin and eosin (H&E) staining and periodic acid-Schiff (PAS) staining were applied to observe the degree of pathological injury and fibrosis of renal tissues. The results of the present study revealed that circ-ITCH expression was obviously decreased in HG-induced RMCs. In addition, circ-ITCH overexpression inhibited the viability, migration, fibrosis and inflammatory response of HG-induced RMCs. Further experiments confirmed that miR-33a-5p may be a direct target of circ-ITCH and SIRT6 may be a direct target of miR-33a-5p. Notably, the miR-33a-5p mimic or shRNA-SIRT6 were discovered to reverse the inhibitory effects of circ-ITCH on the proliferation, migration, fibrosis and inflammatory response of HG-induced RMCs. Furthermore, circ-ITCH overexpression ameliorated renal inflammation and fibrosis in STZ-induced diabetic mice. In conclusion, circ-ITCH alleviated renal inflammation and fibrosis in STZ-induced diabetic mice by regulating the miR-33a-5p/SIRT6 axis.Author names: Please confirm if the author names are presented accurately and in the correct sequence (given name, middle name/initial, family name). Author 1 Given name: [ChunYang] Last name [Xu]. Author 2 Given name: [DingBo] Last name [Xu]. Author 3 Given name: [YongHua] Last name [Liu]. Author 4 Given name: [Juanjuan] Last name [Jiang]. Also, kindly confirm the details in the metadata are correct.ok






Similar content being viewed by others
Availability of data and material
The experimental data will be available on the request.
References
Kato M, Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat Rev Nephrol. 2019;15:327–45.
Lan HY. Transforming growth factor-β/Smad signalling in diabetic nephropathy. Clin Exp Pharmacol Physiol. 2012;39:731–8.
Kashgarian M, Fogo AB. Diagnostic atlas of renal pathology. 3rd ed. Amsterdam: Elsevier; 2017.
Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91.
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P, et al. CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer. 2017;16:94.
Yin Y, Long J, He Q, Li Y, Liao Y, He P, et al. Emerging roles of circRNA in formation and progression of cancer. J Cancer. 2019;10:5015–21.
Liu Y, Yang Y, Wang Z, Fu X, Chu XM, Li Y, et al. Insights into the regulatory role of circRNA in angiogenesis and clinical implications. Atherosclerosis. 2020;298:14–26.
Tian J, Fu Y, Li Q, Xu Y, Xi X, Zheng Y, et al. Differential expression and bioinformatics analysis of CircRNA in PDGF-BB-induced vascular smooth muscle cells. Front Genet. 2020;11:530.
Yan Q, He X, Kuang G, Ou C. CircRNA cPWWP2A: an emerging player in diabetes mellitus. J Cell Commun Signal. 2020;14:351–3.
Yang F, Chen Y, Xue Z, Lv Y, Shen L, Li K, et al. High-throughput sequencing and exploration of the lncRNA-circRNA-miRNA-mRNA network in Type 2 diabetes mellitus. Biomed Res Int. 2020;2020:8162524.
Peng F, Gong W, Li S, Yin B, Zhao C, Liu W, et al. circRNA_010383 acts as a sponge for miR-135a and its downregulated expression contributes to renal fibrosis in diabetic nephropathy. Diabetes. 2020. https://doi.org/10.2337/db200203.
Zhou B, Yu JW. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem Biophys Res Commun. 2017;487:769–75.
Hu W, Han Q, Zhao L, Wang L. Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-β1. J Cell Physiol. 2019;234:1469–76.
Zhou L, Li FF, Wang SM. Circ-ITCH restrains the expression of MMP-2, MMP-9 and TNF-α in diabetic retinopathy by inhibiting miR-22. Exp Mol Pathol. 2021;118:104594.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E1339–46.
Yamashita H, Nagai Y, Takamura T, Nohara E, Kobayashi K. Thiazolidinedione derivatives ameliorate albuminuria in streptozotocin-induced diabetic spontaneous hypertensive rat. Metabolism. 2002;51:403–8.
Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045.
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81.
Cho NH. Q&A: five questions on the 2015 IDF diabetes atlas. Diabetes Res Clin Pract. 2016;115:157–9.
Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016;375:905–6.
Wang ST, Liu LB, Li XM, Wang YF, Xie PJ, Li Q, et al. Circ-ITCH regulates triple-negative breast cancer progression through the Wnt/β-catenin pathway. Neoplasma. 2019;66:232–9.
Ren C, Liu J, Zheng B, Yan P, Sun Y, Yue B. The circular RNA circ-ITCH acts as a tumour suppressor in osteosarcoma via regulating miR-22. Artif Cells Nanomed Biotechnol. 2019;47:3359–67.
Hu J, Wang L, Chen J, Gao H, Zhao W, Huang Y, et al. The circular RNA circ-ITCH suppresses ovarian carcinoma progression through targeting miR-145/RASA1 signaling. Biochem Biophys Res Commun. 2018;505:222–8.
Guo W, Zhang J, Zhang D, Cao S, Li G, Zhang S, et al. Polymorphisms and expression pattern of circular RNA circ-ITCH contributes to the carcinogenesis of hepatocellular carcinoma. Oncotarget. 2017;8:48169–77.
Feng Y, Qu X, Chen Y, Feng Q, Zhang Y, Hu J, et al. MicroRNA-33a-5p sponges to inhibit pancreatic β-cell function in gestational diabetes mellitus LncRNA DANCR. Reprou Biol Endocrinol. 2020;18:61.
Coskun ZM, Beydogan AB, Bolkent S. Changes in the expression levels of CB1 and GLP-1R mRNAs and microRNAs 33a and 122 in the liver of type 2 diabetic rats treated with ghrelin. J Biochem Mol Toxicol. 2019;33:e22388.
Xu BH, Sheng J, You YK, Huang XR, Ma RCW, Wang Q, et al. Deletion of Smad3 prevents renal fibrosis and inflammation in type 2 diabetic nephropathy. Metabolism. 2020;103:154013.
Huang H, Ni H, Ma K, Zou J. ANGPTL2 regulates autophagy through the MEK/ERK/Nrf-1 pathway and affects the progression of renal fibrosis in diabetic nephropathy. Am J Transl Res. 2019;11:5472–86.
Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, et al. LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8:e2583.
Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124:139–52.
Chen Z, Ding HS, Guo X, Shen JJ, Fan D, Huang Y, et al. MiR-33 promotes myocardial fibrosis by inhibiting MMP16 and stimulating p38 MAPK signaling. Oncotarget. 2018;9:22047–57.
Erhartova D, Cahova M, Dankova H, Heczkova M, Mikova I, Sticova E, et al. Serum miR-33a is associated with steatosis and inflammation in patients with non-alcoholic fatty liver disease after liver transplantation. PLoS ONE. 2019;14:e0224820.
Vega-Badillo J, Gutiérrez-Vidal R, Hernández-Pérez HA, Villamil-Ramírez H, León-Mimila P, Sánchez-Muñoz F, et al. Hepatic miR-33a/miR-144 and their target gene ABCA1 are associated with steatohepatitis in morbidly obese subjects. Liver Int. 2016;36:1383–91.
Ji L, Chen Y, Wang H, Zhang W, He L, Wu J, et al. Overexpression of Sirt6 promotes M2 macrophage transformation, alleviating renal injury in diabetic nephropathy. Int J Oncol. 2019;55:103–15.
Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, et al. Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation. Int J Biol Sci. 2019;15:701–13.
Muraoka H, Hasegawa K, Sakamaki Y, Minakuchi H, Kawaguchi T, Yasuda I, et al. Role of nampt-Sirt6 axis in renal proximal tubules in extracellular matrix deposition in diabetic nephropathy. Cell Rep. 2019;27:199-212.e5.
Funding
National Natural Science Foundation of China (Project No. 81760153); Key R & D Program of Jiangxi Province (Project No. 20181BBG70014); Key project of Key R & D Program of Jiangxi Province (Project No. 20171ACH80002).
Author information
Authors and Affiliations
Contributions
JL: project development, literature search, data analysis and collection, manuscript writing. PD: project development, literature search, data analysis and collection, manuscript writing. CX: project development, data collection. DX: project development, data collection. YL: project development, data collection. JJ: project development, manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare they have no competing interests.
Ethics approval and consent to participate
Animal experiments were approved by the Ethics Committee of Chinese PLA General Hospital.
Consent for publication
Not applicable.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Duan, P., Xu, C. et al. CircRNA circ-ITCH improves renal inflammation and fibrosis in streptozotocin-induced diabetic mice by regulating the miR-33a-5p/SIRT6 axis. Inflamm. Res. 70, 835–846 (2021). https://doi.org/10.1007/s00011-021-01485-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01485-8