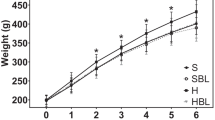
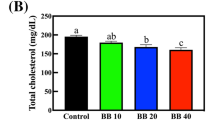
Abstract
Time-restricted feeding and food enriched in polyphenols are strategies to prevent or reduce metabolic disorders. Bean leaves (Phaseolus vulgaris L.) are a recognized source of polyphenolic compounds, whose effects on metabolic pathways are not well studied. We evaluated the combined effects of dietary supplementation with Phaseolus vulgaris leaves (10% w/w) (BL) and a 7-h daytime-restricted feeding protocol (RF) under a hypercaloric diet (high fat + high fructose) (HFFD) on the metabolic parameters related to glucose and lipid handling. Adult male Wistar rats were treated for 8 weeks with standard and HFFD diets with or without BL. The results showed that RF improved metabolic alterations induced by HFFD (e.g., hepatic steatosis, increased triacylglycerols, and serum lipoproteins). Supplementation with BL significantly enhanced this effect and downregulated the mRNA expression of carbohydrate and lipid metabolism genes in the liver. These results indicate that dietary supplementation with BL enhances the benefits elicited by RF.



Similar content being viewed by others
Abbreviations
- Acc:
-
Acetyl-CoA carboxylase
- BL:
-
Bean leaves
- Fas:
-
Fatty acid synthase
- G6pase:
-
Glucose-6-phosphatase
- G6pd:
-
Glucose-6-phosphate dehydrogenase
- Gk:
-
Glucokinase
- HDL:
-
High-density lipoprotein
- HFFD:
-
High fat + high fructose diet
- HOMA-IR:
-
Homeostatic Model Assessment for Insulin Resistance
- LDL:
-
Low-density lipoprotein
- Pepck:
-
Phosphoenolpyruvate carboxykinase
- Pklr:
-
Pyruvate kinase
- RF:
-
Restricted feeding
- Scd1:
-
Stearoyl-CoA desaturase-1
- SD:
-
Standard diet
- TAG:
-
Triacylglycerides
- VLDL:
-
Very-low-density lipoprotein
References
Vileigas DF, Marciano CLC, Mota GAF et al (2019) Temporal measures in cardiac structure and function during the development of obesity induced by different types of western diet in a rat model. Nutrients 12:68. https://doi.org/10.3390/nu12010068
Hatori M, Vollmers C, Zarrinpar A et al (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15:848–860. https://doi.org/10.1016/j.cmet.2012.04.019
Chaix A, Lin T, Le HD et al (2019) Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab 29:303-319.e4. https://doi.org/10.1016/j.cmet.2018.08.004
Hay E, Lucariello A, Contieri M et al (2019) Therapeutic effects of turmeric in several diseases: an overview. Chem Biol Interact 310:108729. https://doi.org/10.1016/j.cbi.2019.108729
Zeni ALB, Moreira TD, Dalmagro AP et al (2017) Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An Acad Bras Cienc 89:2805–2815. https://doi.org/10.1590/0001-3765201720160660
Donado-Pestana CM, Dos Santos-Donado PR, Daza LD et al (2018) Cagaita fruit (Eugenia dysenterica DC.) and obesity: role of polyphenols on already established obesity. Food Res Int 103:40–47. https://doi.org/10.1016/j.foodres.2017.10.011
Ganesan K, Xu B (2017) Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int J Mol Sci 18:2331. https://doi.org/10.3390/ijms18112331
Quiroz-Sodi M, Mendoza-Díaz S, Hernández-Sandoval L et al (2018) Characterization of the secondary metabolites in the seeds of nine native bean varieties (Phaseolus vulgaris and P. Coccineus) from Querétaro, Mexico. Bot Sci 96:650–661. https://doi.org/10.17129/botsci.1930
Wortmann CS, Kirkby RA, Eledu CA et al (1998) Atlas of common bean (Phaseolus vulgaris L.) production in Africa. Centro Internacional de Agricultura Tropical (CIAT), Cali, CO. p. 131 (CIAT publication no. 297)
Martínez-Zavala M, Mora-Avilés MA, Anaya-Loyola MA et al (2016) Common bean leaves as a source of dietary iron: functional test in an iron-deficient rat model. Plant Foods Hum Nutr 71:259–264. https://doi.org/10.1007/s11130-016-0554-5
Layman JI, Pereira DL, Chellan N et al (2019) A histomorphometric study on the hepatoprotective effects of a green rooibos extract in a diet-induced obese rat model. Acta Histochem 121:646–656. https://doi.org/10.1016/j.acthis.2019.05.008
Kumar N, Goel N (2019) Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst) 24:e00370. https://doi.org/10.1016/j.btre.2019.e00370
Sun S, Hanzawa F, Kim D et al (2019) Circadian rhythm–dependent induction of hepatic lipogenic gene expression in rats fed a high-sucrose diet. J Biol Chem 294:15206–15217. https://doi.org/10.1074/jbc.RA119.010328
Gabbia D, Roverso M, Guido M et al (2019) Western diet-induced metabolic alterations affect circulating markers of liver function before the development of steatosis. Nutrients 11:1602. https://doi.org/10.3390/nu11071602
Millar CL, Duclos Q, Blesso CN (2017) Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr 8:226–239. https://doi.org/10.3945/an.116.014050
Lee S, Lee J, Lee H et al (2019) Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J Food Biochem 43:e13002. https://doi.org/10.1111/jfbc.13002
Yang L, Li XF, Gao L et al (2012) Suppressive effects of quercetin-3-O-(6″-Feruloyl)-β-D-galactopyranoside on adipogenesis in 3T3-L1 preadipocytes through down-regulation of PPARγ and C/EBPα expression. Phytother Res 26:438–444. https://doi.org/10.1002/ptr.3604
Ramalingam S, Karuppiah M, Thiruppathi M et al (2020) Antioxidant potential of biflavonoid attenuates hyperglycemia by modulating the carbohydrate metabolic enzymes in high fat diet/streptozotocin induced diabetic rats. Redox Rep 25:1–10. https://doi.org/10.1080/13510002.2020.1722914
Wu T, Guo Y, Liu R et al (2016) Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct 7:2469–2478. https://doi.org/10.1039/C6FO00401F
Aguilar-Roblero R, Díaz-Muñoz M (2010) Chronostatic adaptations in the liver to restricted feeding: the FEO as an emergent oscillator. Sleep Biol Rhythms 8:9–17. https://doi.org/10.1111/j.1479-8425.2009.00415.x
García-Gaytán AC, Miranda-Anaya M, Turrubiate I et al (2020) Synchronization of the circadian clock by time-restricted feeding with progressive increasing calorie intake. Resemblances and differences regarding a sustained hypocaloric restriction. Sci Rep 10:10036. https://doi.org/10.1038/s41598-020-66538-0
Ding S, Jiang J, Zhang G et al (2017) Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One 12:e0183541. https://doi.org/10.1371/journal.pone.0183541
Kim Y, Keogh JB, Clifton PM (2016) Polyphenols and glycemic control. Nutrients 8:17. https://doi.org/10.3390/nu8010017
Lee Y, Kim EK (2013) AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Exp Mol Med 45:e33. https://doi.org/10.1038/emm.2013.65
Acknowledgements
We thank Jéssica González Norris for assistance with the English version of this manuscript, and Dr. Christian Molina Aguilar, Dr. Aracely Anaya-Loyola, Nut. Sonia Hernández-Nájera, Nut. Fernando López-Barrera, Lilia Ruíz-Garduño, Biol. Josué López-Martínez, Ing. Nydia Hernández-Ríos, and Nut. Adriana Becerril-Campos for their technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that are no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramírez-Venegas, G., De Ita-Pérez, D., Díaz-Muñoz, M. et al. Supplementation with Phaseolus vulgaris Leaves Improves Metabolic Alterations Induced by High-Fat/Fructose Diet in Rats Under Time-Restricted Feeding. Plant Foods Hum Nutr 76, 297–303 (2021). https://doi.org/10.1007/s11130-021-00904-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-021-00904-9