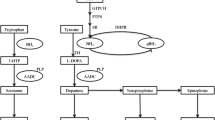
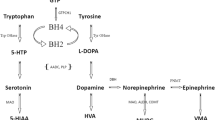
Abstract
Monoamine neurotransmitter disorders present predominantly with neurologic features, including dystonic or dyskinetic cerebral palsy and movement disorders. Genetic conditions that lead to secondary defects in the synthesis, catabolism, transport, and metabolism of biogenic amines can lead to neurotransmitter abnormalities, which can present with similar features. Eleven patients with secondary neurotransmitter abnormalities were enrolled between 2011 and 2015. All patients underwent research-based whole exome and/or whole genome sequencing (WES/WGS). A trial of treatment with levodopa/carbidopa and 5-hydroxytryptophan was initiated. In six families with abnormal neurotransmitter profiles and neurological phenotypes, variants in known disease-causing genes (KCNJ6, SCN2A, CSTB in 2 siblings, NRNX1, KIF1A and PAK3) were identified, while one patient had a variant of uncertain significance in a candidate gene (DLG4) that may explain her phenotype. In 3 patients, no compelling candidate genes were identified. A trial of neurotransmitter replacement therapy led to improvement in motor and behavioral symptoms in all but two patients. The patient with KCNJ6 variant did not respond to L-dopa therapy, but rather experienced increased dyskinetic movements even at low dose of medication. The patient’s symptoms harboring the NRNX1 deletion remained unaltered. This study demonstrates the utility of genome-wide sequencing in further understanding the etiology and pathophysiology of neurometabolic conditions, and the potential of secondary neurotransmitter deficiencies to serve as novel therapeutic targets. As there was a largely favorable response to therapy in our case series, a careful trial of neurotransmitter replacement therapy should be considered in patients with cerebrospinal fluid (CSF) monoamines below reference range.


Similar content being viewed by others
Abbreviations
- 5HIAA:
-
5-Hydroxyindoleacetic acid
- 5HTP:
-
5-Hydroxytryptophan
- AADC:
-
Aromatic amino acid decarboxylase
- ACMG:
-
American College of Medical Geneticists
- BH4 :
-
Tetrahydrobiopterin
- DAT:
-
Dopamine transporter
- CP:
-
Cerebral palsy
- ERK:
-
Extracellular signal-regulated kinase
- HVA:
-
Homovanillic acid
- IDD:
-
Intellectual and developmental disabilities
- IF:
-
Incidental finding
- iNTD:
-
International Working Group on Neurotransmitter Related Disorders
- mtDNA:
-
Mitochondrial DNA
- NT:
-
Neurotransmitter
- PAK:
-
P21-activated kinase
- PD:
-
Parkinson’s disease
- PKC:
-
Protein kinase C
- RAF:
-
Rapidly accelerated fibrosarcoma
- RAS:
-
Rat sarcoma
- SERT:
-
Serotonin transporter
- TH:
-
Tyrosine hydroxylase
- TPH:
-
Tryptophan hydroxylase
- VUS:
-
Variant of uncertain significance
- WES:
-
Whole exome sequencing
- WGS:
-
Whole genome sequencing
References
Purves D, Augustine GJ, Fitzpatrick D, et al (2001) Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10799/
Ng J, Papandreou A, Heales SJ, Kurian MA (2015) Monoamine neurotransmitter disorders - clinical advances and future perspectives. Nat Rev Neurol. https://doi.org/10.1038/nrneurol.2015.172
Mercimek-Mahmutoglu S, Sidky S, Hyland K, Patel J, Donner J, Logan W, Mendoza-Londono R, et al (2015) Prevalence of inherited neurotransmitter disorders in patients with movement disorders and epilepsy: a retrospective cohort study. Orphanet Rare Dis 10(1). https://doi.org/10.1186/s13023-015-0234-9
De Grandis E, Serrano M, Pérez-Dueñas B, Ormazábal A, Montero R, Veneselli E, Pineda M et al (2010) Cerebrospinal fluid alterations of the serotonin product, 5-hydroxyindolacetic acid, in neurological disorders. J Inherit Metab Dis 33(6):803–809. https://doi.org/10.1007/s10545-010-9200-9
Molero-Luis M, Serrano M, Ormazábal A, Pérez-Dueñas B, García-Cazorla À, Pons R, Artuch R (2013) Homovanillic acid in cerebrospinal fluid of 1388 children with neurological disorders. Dev Med Child Neurol 55(6):559–566. https://doi.org/10.1111/dmcn.12116
Matthews AM, Blydt-Hansen I, Al-Jabri B, Andersen J, Tarailo-Graovac M, Price M, Selby K et al (2019) Atypical cerebral palsy: genomics analysis enables precision medicine. Genet Med 21(7):1621–1628. https://doi.org/10.1038/s41436-018-0376-y
Van Der Heyden JC, Rotteveel JJ, Wevers RA (2003) Decreased homovanillic acid concentrations in cerebrospinal fluid in children without a known defect in dopamine metabolism. Eur J Paediatr Neurol 7(1):31–37. https://doi.org/10.1016/S1090-3798(02)00137-X
Devinsky O, Emoto S, Goldstein DS, Stull R, Porter RJ, Theodore WH, Suzan Nadi N (1992) Cerebrospinal fluid and serum levels of DOPA, catechols, and monoamine metabolites in patients with epilepsy. Epilepsia 33(2):263–270. https://doi.org/10.1111/j.1528-1157.1992.tb02315.x
García-Cazorla A, Serrano M, Pérez-Dueñas B, González V, Ormazábal A, Pineda M, Fernández-álvarez E, Campistol JMD, Artuch RMD (2007) Secondary abnormalities of neurotransmitters in infants with neurological disorders. Dev Med Child Neurol 49(10):740–744. https://doi.org/10.1111/j.1469-8749.2007.00740.x
Ramaekers V, Th J, Senderek M, Häusler M, Häring N, Abeling K, Zerres C, Bergmann GH, Blau N (2001) A novel neurodevelopmental syndrome responsive to 5-hydroxytryptophan and carbidopa. Mol Genet Metab 73(2):179–187. https://doi.org/10.1006/mgme.2001.3187
Horvath GA, Demos M, Shyr C, Matthews A, Zhang L, Race S, Stockler-Ipsiroglu S et al (2016) Secondary neurotransmitter deficiencies in epilepsy caused by voltage-gated sodium channelopathies: a potential treatment target? Mol Genet Metab 117(1):42–48. https://doi.org/10.1016/j.ymgme.2015.11.008
Tarailo-Graovac M, Shyr C, Ross CJ, Horvath GA, Salvarinova R, Ye XC, Zhang L-H et al (2016) Exome sequencing and the management of neurometabolic disorders. N Engl J Med 374(23):2246–2255. https://doi.org/10.1056/NEJMoa1515792
Van Karnebeek CDM, Shevell M, Zschocke J, Moeschler JB, Stockler S (2014) The metabolic evaluation of the child with an intellectual developmental disorder: diagnostic algorithm for identification of treatable causes and new digital resource. Mol Genet Metab. https://doi.org/10.1016/j.ymgme.2014.01.011
Hyland K (2003) The lumbar puncture for diagnosis of pediatric neurotransmitter diseases. Ann Neurol 54(S6):S13-17. https://doi.org/10.1002/ana.10627
Lehman A, Thouta S, Mancini G, Naidu S, VanSlegtenhorst M, McWalter K et al (2017) Loss-of-Function and gain-of-function mutations in KCNQ5 cause intellectual disability or epileptic encephalopathy. Am J Hum Genet 101(1):65–74
Horvath GA, Tarailo-Graovac M, Bartel T, Race S, Van Allen MI, Blydt-Hansen I, Ross CJ, Wasserman WW, Connolly MB, van Karnebeek CDM (2018) Improvement of self-injury with dopamine and serotonin replacement therapy in a patient with a hemizygous PAK3 mutation: a new therapeutic strategy for neuropsychiatric features of an intellectual disability syndrome. J Child Neurol 33(1):106–113. https://doi.org/10.1177/0883073817740443
Horvath G, Zhao Y, Tarailo-Graovac M, Boelman C, Gill H, Shyr C, Lee J, Blydt-Hansen I, Drogemoller B, Moreland J, Ross C, Wasserman W, Masotti A, Slesinger P, van Karnebeek C (2018) Gain-of-function KCNJ6 mutation in a severe hyperkinetic movement disorder phenotype. Neuroscience 384:152–164. https://doi.org/10.1016/j.neuroscience.2018.05.031
Lelieveld SH, Reijnders MRF, Pfundt R, Yntema HG, Kamsteeg EJ, De Vries P, De Vries BBA et al (2016) Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat Neurosci 19(9):1194–1196. https://doi.org/10.1038/nn.4352
Xing J, Kimura H, Wang C, Ishizuka K, Kushima I, Arioka Y, Yoshimi A et al (2016) Resequencing and association analysis of six PSD-95-related genes as possible susceptibility genes for schizophrenia and autism spectrum disorders. Sci Rep 6(1):1–8. https://doi.org/10.1038/srep27491
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomic and the Association for Molecular Pathology. Genet Med 17:405–423
Marecos C, Ng J, Kurian MA (2014) What is new for monoamine neurotransmitter disorders? J Inherit Metab Dis 37(4):619–26. https://doi.org/10.1007/s10545-014-9697-4
Kurian MA, Gissen P, Smith M, Heales SJr, Clayton PT (2011) The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. The Lancet 10(8):721–733
Ng J, Tuschl K, Csanyi B, Kinali M, Devlin A, Carr LJ, Cleary M et al (2013) TH-negative infantile-onset severe dopamine deficiency syndrome. Eur J Paediatr Neurol 17:S128. https://doi.org/10.1016/s1090-3798(13)70452-5
Garcia-Cazorla A, Duarte S, Serrano M, Nascimento A (2008) Mitochondrial diseases mimicking neurotransmitter defects. Mitochondrion 8(3):273–278
Van Karnebeek CDM, Dunbar M, Egri C, Sayson B, Milea J, Stockler-Ipsiroglu S, Huh L, Connolly MB, Horvath GA (2018) Secondary abnormal csf neurotransmitter metabolite profiles in a pediatric tertiary care centre. Can J Neurol Sci 45(2):206–213. https://doi.org/10.1017/cjn.2017.271
Brady ST, Albers RW, Siegel GJ, Price DL (2012) Basic neurochemistry: principles of molecular, cellular, and medical neurobiology, 8th edn. Academic Press, Amsterdam. https://doi.org/10.1016/C2009-0-00066-X
Bevensee MO (2014) Current topics in membranes. Exchangers. Vol. 73. Academic Press
Shin OH (2014) Exocytosis and synaptic vesicle function. Compr Physiol 4(1):149–175. https://doi.org/10.1002/cphy.c130021
Wolf M, Zimmermann AM, Görlich A, Gurniak C, Sassoe-Pognetto M, Friauf E, Witke W, Rust M (2015) ADF/cofilin controls synaptic actin dynamics and regulates synaptic vesicle mobilization and exocytosis. Cereb Cortex 25(9):2863–2875
Daubner SC, Le T, Wang S (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch Biochem Biophys 508(1):1–12
Joensuu T, Lehesjoki AE, Kopra O (2008) Molecular background of EPM1 - Unverricht-Lundborg Disease. Epilepsia. https://doi.org/10.1111/j.1528-1167.2007.01422.x
Vaarmann A, Kaasik A, Zharkovsky A (2006) Altered tryptophan metabolism in the brain of cystatin b-deficient mice: a model system for progressive myoclonus epilepsy. Epilepsia 47(10):1650–1654. https://doi.org/10.1111/j.1528-1167.2006.00638.x
Puschmann A, Wszolek ZK, Farrer M et al (2009) Alpha-synuclein multiplications with Parkinsonism, dementia or progressive myoclonus? Parkinsonism Relat Disord 15(5):390–392
Gondi CS, Kandhukuri N, Kondraganti S, Gujrati M, Olivero WC, Dinh DH, Rao JS (2006) Down-regulation of UPAR and cathepsin B retards cofilin dephosphorylation. Int J Oncol 28(3):633–39
Horvath G, Meisner L, Selby K, Stowe R, Carleton B (2017) Improved strength on 5-hydroxytryptophan and carbidopa in spinal cord atrophy. J Neurol Sci 378:59–62. https://doi.org/10.1016/j.jns.2017.04.047
Opladen T, Cortes-Saladelafont E, Mastrangelo M, Horvath G, Pons R, Lopez-Laso E, Fernandez-Ramos J, Honzik T, Pearson T, Fredman J, Scholl-Burgi S, Wassenburg T, Jung-Klawitter S, Kuseyri O, Jeltsch K, Kurian M, Garcia-Cazorla A (2016) The international working group on neurotransmitter related disorders (iNTD): a worldwide research project focused on primary and secondary neurotransmitter disorders. Mol Genet Metab Rep 9:61–66. https://doi.org/10.1016/j.ymgmr.2016.09.006
Acknowledgements
We are indebted to all patients and their families for participation in this study; our colleagues in the Departments of Pediatrics and Medical Genetics, University of British Columbia (Canada) for clinical management of patients; and other colleagues at BC Children’s Hospital and Vancouver General Hospital contributing to the diagnoses and management of these patients.
Funding
This study is supported by funding from the B.C. Children’s Hospital Foundation (Treatable Intellectual Disability Endeavour in British Columbia: 1st Collaborative Area of Innovation www.tidebc.org); Genome BC (SOF-195 grant to CvK); BC Clinical Genomics Network (#00032 grant to CvK); Rare Diseases Foundation (microgrants to CvK & GH), Canadian Institutes of Health Research (#301221 grant to CvK, RDMM), Michael Smith Foundation for Health Research Scholar Award (to CvK), Genome Canada (ABC4DE Project to WW; RDMM); CIHR New Investigator Award (to CR); CFRI Fellowship to AM; CFRI PhD award to JL and Alberta Children’s Hospital Research Institute Foundation.
The CAUSES Study was funded by the BC Children’s Hospital Foundation (Mining for Miracles) and GenomeBC; investigators include Shelin Adam, Christele Du Souich, Alison Elliott, Anna Lehman, Jill Mwemifumbo, Tanya Nelson, Clara van Karnebeek and Jan Friedman. Bioinformatics support was provided to CAUSES by the laboratory of Wyeth Wasserman.
Author information
Authors and Affiliations
Consortia
Contributions
All authors made substantial contributions to the conception or design of the work (GAH, MTG, CvK) and the acquisition, analysis, or interpretation of data (MTG, IBH, AM, VA, MP, BD, CS, JL, JM, AG, SS, JMF, AL, CR, WW). GAH, MTG, IBH drafted the manuscript. CvK, JMF, AL revised it critically for important intellectual content, and all authors approved the current version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Table 3
Presenting symptoms of patients. (DOCX 16 KB)
Rights and permissions
About this article
Cite this article
van Karnebeek, C.D., Blydt-Hansen, I., Matthews, A.M. et al. Secondary biogenic amine deficiencies: genetic etiology, therapeutic interventions, and clinical effects. Neurogenetics 22, 251–262 (2021). https://doi.org/10.1007/s10048-021-00652-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-021-00652-7