Abstract
A new species of the sea pen genus Scytalium Herklots, 1858, is proposed based on the morphological and molecular study of a colony collected during the Océano Profundo 2018 cruise to the Caribbean Sea (NW Atlantic). The new species, Scytalium herklotsi sp. nov., is characterized by its well-developed and overlapping polyp leaves (up to 68 mm in length and 27 mm in width), the presence of eight spiculated large digitiform processes at the distal part of the basal portion of autozooids, the axis rounded in cross section, the numerous siphonozooids in a continuous band of 3–6 rows along the latero-dorsal area of the rachis, and up to 3 rows between contiguous polyp leaves, among other features. An additional unrecorded type of sclerites is reported from the pharynx of autozooids, probably also present in other species of the genus. Scytalium herklotsi sp. nov. is the first species of the genus recorded so far from the Atlantic Ocean, all other described species being Indo-West Pacific. The new species is also the deepest record of a Scytalium species (ca 500 m in depth); the previous published records range from 10 to 206 m in depth. A molecular comparison based on a set of concatenated sequences of four markers (three mitochondrial genes (mtMutS, ND2, and Cox1) and a nuclear segment (28S)) relates the new species to other published sequences attributed to Scytalium species, within the named Clade II of previous general phylogenetic studies on the octocoral Order Pennatulacea.
Similar content being viewed by others
Introduction
The genus Scytalium Herklots, 1858 was described by Herklots (1858) for the type species S. sarsii Herklots, 1858. Although in the original description, the origin of the specimen in the collection of Leiden museum was mentioned in a very vague way (Mers du nord), subsequent discussions and findings identified it as a species from the Indian Ocean (Kölliker 1870, 1880).
Kölliker (1870, 1880) described the second and third species in this genus, Scytalium martensii Kölliker, 1870 in Kölliker 1869-72 and S. tentaculatum Kölliker, 1880, respectively, based mainly on a large number of polyps per polyp leaf and larger sclerites in the former, and the presence of a large pointed horn-like process in the proximal part of the autozooids (“calyx”) of the latter. Thomson and Henderson (1906) described two species of Pennatula (P. splendens Thomson & Henderson, 1906, and P. veneris Thomson & Henderson, 1906), which Balss (1909, p. 428) later transferred to the genus Scytalium. Finally, Hickson (1916) described the last species in this genus, Scytalium balssii Hickson, 1916, and discussed the validity of previous species. For this, he reported materials and characters used in the delimitation of the described species, also including the only available key to species in the genus.
According to Williams (1995), at least three of the six nominal species could be valid (S. sarsii, S. martensii, and S. tentaculatum), whereas Williams (1999) increased this possible list to four (considering also S. balssii as valid). Cordeiro et al. (2021) considered Scytalium veneris (Thomson & Henderson, 1906) as uncertain while the status of another species (S. splendens) became controversial (considered a synonym of S. martensii by Kükenthal and Broch (1911, p. 310) when it was reported by Imahara and Namikawa 2018). Despite the small number of species described, the limits between some of them are still not clear enough, a fact that is not helped by the erections of varieties (Thomson and Simpson 1909).
Against this background, the molecular study by Kushida and Reimer (2018, p. 237) identified Scytalium as a monophyletic genus within Clade II proposed by Dolan et al. (2013). It became positioned far from other proposed virgulariid partners (genera Virgularia Lamarck, 1816, and Stylatula Verrill, 1864 (see Kushida and Reimer 2018), or Acanthoptilum Kölliker, 1870 in Kölliker 1869-72 (see García-Cárdenas et al. 2020)). Instead of the traditional systematic relationship based on morphological characters, Scytalium appeared to be strongly related to a specimen identified as Pennatula sp., which deserved further research. Despite the recognition of the polyphyly of the family Virgulariidae, mitochondrial markers showed enough genetic variability that can be used in the search for species delimitation.
During a recent expedition exploring deep-sea bottoms near Puerto Rico (Caribbean Sea), an elongated and delicate sea pen was collected by an ROV (remotely operated vehicle). The present paper describes that colony based on a complementary morphological analysis (using light and SEM microscopy) and molecular methods (sequencing of three mitochondrial and one nuclear markers). The result of this study identifies the specimen as a species of the genus Scytalium. Different morphological and genetic features allow the description of a new species; the first one found with certainty in the Atlantic Ocean, as well as the emendation of the generic diagnosis.
Material and methods
The specimen described here was obtained during the Océano Profundo 2018 expedition, carried out by the NOAA Research Vessel Okeanos Explorer, and collected by the ROV Deep Discoverer (also of NOAA) (Wagner et al. 2019). The colony was fixed on board in ethanol 95% for morphological examination, and further molecular studies. Sclerites from different colony parts were prepared for an SEM study employing the standard methodology described by, for example, Bayer and Stefani (1988). Permanent mounts were made for light microscopy. Colony and sclerite terminology follow Bayer et al. (1983). Fragments of the ventral edge of the polyp leaves with polyps were prepared using an adapted hexamethyldisilazane (HMDS) protocol, limiting the immersion of the octocoral fragments in this chemical to 3 min (Nation 1983; Braet et al. 1997; Shively and Miller 2009; López-González 2020). These fragments were mounted on stubs, coated with gold-palladium under a Leica ACE600, and observed with a Zeiss EVO SEM at the General Research Services of Microscopy at the University of Seville. The axis was sectioned, and polished using 600, 800, 1200, and 3000 diamond polishing discs on a mini-cutting/polishing table YXEC. Cross sections were visualized and photographed under UV light (SFA-UV Stereomicroscope Adapter NIGHTSEA) using a stereomicroscope Motic SMZ-168.
Total genomic DNA was extracted from the ethanol (EtOH)-preserved specimen using the E.Z.N.A. DNA kit (OmegaBiotech) following the manufacturer’s instructions. Three mitochondrial regions, mtMutS (=msh1), ND2 and Cox1, plus a nuclear region (28S ribosomal DNA) were sequenced. These four markers were concatenated, representing the largest multiloci segment used today in sea pen phylogenies, previous contributions used mtMutS+ND2 (Dolan et al. 2013; Kushida and Reimer 2018) or mtMutS+Cox1+28S (García-Cárdenas et al. 2020). The start of the mtMutS region was amplified using the primers ND42599F and MUT3458R (France and Hoover 2002; Sánchez et al. 2003). The star of the ND2 region was amplified using the primers 16S47F and ND2-1418R (McFadden et al. 2004). Each PCR used 1 U of MyTaq Red DNA Polymerase (Bioline), 10 μM of each primer, approximately 30 ng of genomic DNA, and was brought to a final volume of 25 μL with H2O for molecular biology (PanReac-AppliChem). MtMutS PCR was carried out using the following cycle profile: initial denaturation at 95 °C for 1 min, 35 cycles of denaturation at 95 °C for 15 s, annealing at 55 °C for 15 s, and extension at 72 °C for 10 s, and a final extension at 72 °C for 5 min. The ND2, PCR used the same cycle profile, but 51 °C as the annealing temperature. PCR products were purified using ExoSAP–IT™ PCR Product Cleanup Reagent (ThermoFisher Scientific) following the manufacturer’s instructions, before strong amplifications were sent to Macrogen Spain for sequencing in both directions. All chromatograms were visualized and sequence pairs matched and edited using Sequencher v4.0.
A preliminary BLAST search identified our specimen as belonging to the genus Scytalium Herklots, 1858. This relationship was confirmed in a ML comparison based on mtMutS (ca. 400 pennatulacean sequences, not shown), where the new proposed taxon fell very close to other Scytalium sequences, within an informally named Clade II (see Dolan et al. 2013; Kushida and Reimer 2018). This molecular placement is in agreement with morphological features that will be described below (see “Results”). Therefore, for the present study, we will only include sequences of Clade II in order not to repeat images and ideas already discussed. The current available molecular information concerning this genus covers only mtMutS and ND2 markers (Dolan et al. 2013; Kushida and Reimer 2018).
The set of new sequences obtained in this study and those homologous from GenBank (see Table 1) were aligned using MUSCLE, implemented in MEGA 6 (Tamura et al. 2013). According to previous cited molecular phylogenies and the recent one by García-Cárdenas et al. (2020), the basal resolution of the main sea pen clades is poor. Thus, sequences of two ellisellids from GenBank were selected as an out-group. The aligned mtMutS+ND2+Cox1+28S set of sequences had 38 sequences (36 pennatulaceans and two ellisellids), a total of 2834 positions, with 720 variable and 523 parsimony-informative sites. After alignment, the best nucleotide substitution model was selected using Modeltest implemented in MEGA6, according to Akaike Information Criterion (AIC) and hierarchical likelihood ratio test (hLRT) values. The phylogeny reconstruction was obtained applying Maximum Likelihood (ML) and Bayesian inference (BI) methods. The Maximum Likelihood method was carried out in MEGA6 using the NNI (Nearest Neighbor Interchange) heuristic method and 1000 bootstrap replications. The selected nucleotide substitution model was GTR+G. The Bayesian Inference was carried out with MrBayes v3.1.2 (Huelsenbeck and Ronquist 2001, Ronquist and Huelsenbeck 2003), using the substitution model GTR+G (lset nst=6 rates=gamma), 107 generations, and discarding 25% of the initial trees. The stationary state of the chains and convergence of the two runs were monitored for each parameter by Tracer (v.1.7.1) (Rambaut et al. 2018), determining whether the effective sample size (ESS) of all parameters was larger than 200, as recommended.
Results
Subclass Octocorallia Haeckel, 1866
Order Pennatulacea Verrill, 1865
Family Virgulariidae Verrill, 1868 in Verill 1868-70
Genus Scytalium Herklots, 1858
Scytalium Herklots 1858: 14. Gray 1860: 21; Richiardi 1869: 66; Kölliker 1869-72: 573; Kölliker 1880: 10; J.A. Thomson and Simpson 1909: 282; Balss 1909: 427; Balss 1910: 48; Kükenthal and Broch 1911: 310; Kükenthal 1915: 65; Hickson 1916: 202; Williams 1995: 123; Imahara and Namikawa 2018: 86.
Pennatula. Thompson and Henderson 1906: 115 (in part).
Diagnosis (modified from Williams 1995, p. 123)
Colonies elongated and slender to more stout and robust. Bilateral symmetry throughout rachis. Axis present throughout colony, rounded to quadrangular (but not X-shaped) in cross section. Polyp leaves present, thin and fleshy with the broadest part of each leaf being where it joins with the rachis at a very oblique angle, almost parallel to the axis of the rachis. Autozooids numerous along the ventral margins of polyp leaves, no other type of polyp present on the polyp leaves. Autozooids at least partially retractile into bulbous or tubular basal portions of the polyps, distalmost part of calyces with 0 to 8 processes. Siphonozooids arranged on the rachis between the polyp leaves and along both sides of a naked dorsal track. Platelet sclerites small (< 80 μm in length) as oval-shaped plates, often medially constricted with a distinct waist, sometimes with central surface material partially deciduous. Platelet sclerites distributed in the rachis, peduncle, polyp leaves, proximal portions of autozooids (basal portions and, if present, digitiform processes), as well as occasionally in the tentacles. Tentacles with or without sclerites along aboral side of main tentacular axis. Pharynx with (or without?) minute (~10 μm) white-translucent ovals.
Distribution and depth
Indo-West Pacific (Red Sea, southeastern Africa, Indian Ocean, Malay Archipelago, Philippines, China, Taiwan, Japan) and Atlantic (Caribbean Sea); 10–489 m depth (Williams 1995, 2011; Imahara and Namikawa 2018; present contribution).
Type species
Scytalium sarsii Herklots, 1858 (by monotypy)
Remarks
All recent molecular studies failed to recover the monophyly of the family Virgulariidae (Dolan et al. 2013; Kushida and Reimer 2018; García-Cárdenas et al. 2020). The placement of the genus Scytalium in the family Virgulariidae is here considered for practical reasons, to be in agreement with previous literature, but waiting for a more global revision of the family delimitations within the Pennatulacea.
Scytalium herklotsi sp. nov.
http://zoobank.org/8C22F2C7-A421-4821-B59C-25EDC320861C
Examined material
USNM 1550636, Holotype, North Atlantic Ocean, Caribbean Sea, Puerto Rico, Caja de Muertos, EX1811-Dive 07, 17° 49′ 30″ N 66° 33′ 59″ W, 489.47 m depth, 7 Nov 2018. Coll. D. Wagner.
Morphological description of the holotype
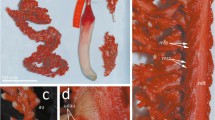
Colony elongated, pinnate, and erect (Fig. 1a) 618 mm in length in preserved state. Rachis bilaterally symmetrical, 530 mm in length (85.6% of overall length) and 4 mm in width (measured at mid-length of rachis, without polyp leaves). Rachis-peduncle limit prominently swollen (Fig. 1a). Peduncle 88 mm in length (14.4% of overall length) and 15 mm in width at the widest point (the limit rachis-peduncle). Rachis with 35 delicate polyp leaves on each side, inserted obliquely, overlapping, almost parallel to the axis of the rachis, and extending ventrally upward (Fig. 1b). Polyp leaves placed alternately (Fig. 1c), increasing in size along the rachis until the mid-zone to last third, then decreasing in size towards the distal part. Rachis with distinctive naked dorsal (about 3 mm width, Fig. 1d) and ventral track (about 1 mm width, Fig. 1e). Polyp leaves’ maximum length 68 mm, maximum width 28 mm. Axis present throughout colony, rounded in cross section (Fig. 1f, g), 2.8 mm in maximum diameter. Autozooids numerous, approximately up to 35 in the largest polyp leaves (plus 4–5 small developing autozooids at the proximal portion of the ventral polyp leaf, see Fig. 1e; see “Discussion”). Autozooids delicate but well developed (up to 5 mm in length, up to 1.4 mm in width), arranged in a single row (Fig. 2a, b). Anthocodiae partially retractile into tubular basal portions of polyps, distalmost part of anthostele with eight hollow digitiform processes (up to 1 mm in length and 0.2 mm in width) aligned with tentacles (Figs. 2b, 3a–d). Autozooids with lines of mesenterial insertions visible (Fig. 2a, b), clearly marked along the polyp body (Fig. 3e). Siphonozooids minute, 0.16–0.21 mm in diameter (average 0.18 mm, n=20), numerous, on the axillae of polyp leaves (Figs. 1d, 2d) and as a band of 3–6 rows along the latero-dorsal surface of rachis. Female, with developing oocytes of up to 0.67 mm in diameter (Fig. 2e, f).
Scytalium herklotsi sp. nov. Holotype (USNM 1550636). a Whole colony, arrows indicate the precedence of the cross sections of axis in f and g; b Detail of well-developed polyps leaves, rachis in lateral view; c Latero-ventral view of a set of overlapping and alternate polyp leaves from the mid-distal part of the rachis showing the coloured autozooids (au); d Latero-dorsal view of the rachis, mid-distal part, showing the continuous vertical band of siphonozooids (vsz), the band of siphonozooids between consecutive polyps leaves (szpl), and the coloured naked dorsal track (ndt); e Naked ventral track (nvt), and developing autozooids at the innermost part of the polyp leaf (black arrows); f Cross section of the axis at rachis-peduncle limit, showing growth rings in a circular pattern (see arrow in a); g Cross section of the axis at distal third of the rachis (see arrow in a). Photos a and b by T. Coffer USNM
Scytalium herklotsi sp. nov. Holotype (USNM 1550636). a Group of autozooids from a mid-rachis polyp leaf; b Detail from a, showing the basal pat of three autozooids, their long digitiform processes (dp) (both coloured by the abundant red platelet sclerites), and white tentacles (te); c Detail of the lateral side of a polyp leaf showing the contiguous long gastrovascular cavities of the autozooids, note the minute red sclerites; d Detail of the longitudinal latero-posterior band of siphonozooids (si) and the minute coloured sclerites around each opening; e Detail of oocytes (oo) throughout the delicate wall of a polyp leaf; f Detail of an oocyte showing nucleolus (nu) and nucleus (nc); g Terminal part of an autozooid cut longitudinally to observe the white tentacles (te), digitiform processes (dp), pharynx (ph), and mesenterial filaments (mf); h Coloured platelets (cp) from the autozooid basal body and minute white bodies (wb) from pharynx; i Detail of the minute white bodies (wb) from pharynx
Scytalium herklotsi sp. nov. Holotype (USNM 1550636). SEM photographs. a Distal portion of an autozooid sectioned longitudinally, showing tentacles with pinnulae (te), digitiform processes (dp), and pharynx (ph); b Transversal section at the upper part of an autozooid, showing the hollow digitiform processes (dp) and tentacles (te); c Detail from b, showing two digitiform processes sectioned transversally and platelet sclerites (ps); d Detail from c, showing platelet sclerites (ps) in the wall of a digitiform process (dp); e Autozooid sectioned transversally at pharynx level, showing pharynx (ph), well-defined mesenterial insertions (mi), and mesenteria (ms)
Sclerites differentially distributed in various parts of the colony. Densely placed in rachis along dorsal and ventral naked tracks (Fig. 1d, e), around siphonozooids openings (Fig. 2d), and basal part of polyps including digitiform processes (Fig. 2a, b). On lateral surface of polyp leaves, sclerites are mainly restricted along the limits between elongated contiguous gastrovascular cavities (Fig. 2c), becoming scarce proximally (Fig. 2e). Lateral surfaces of proximal polyp leaves more densely spiculed, and so dark reddish in colour, instead of the whitish colour of mid and terminal polyp leaves. Sclerite layer in peduncle placed deeper than in rachis, producing a lighter colour than the latter.
Sclerites from basal portion of polyps and digitiform processes up to 0.056 mm in length (Fig. 4a). Sclerites from polyp leaves up to 0.045 mm in length (Fig. 4b). Sclerites from the naked dorsal rachis up to 0.045 mm (Fig. 4c). Sclerites from siphonozooids up to 0.044 mm in length (Fig. 5a). Sclerites from surface area of the rachis/peduncle boundary region up to 0.045 mm in length (Fig. 5b). Sclerites from peduncle up to 0.035 mm in length (Fig. 5c). Minute white bodies from pharynx up to 0.01 mm in length (Fig. 2h, i).
Colour
Preserved colony is whitish, light red to dark red, depending on the density of dark red sclerites (Figs. 1a–d, 2a–e, g). Pharynx is white because of the colour of the minute bodies (Fig. 2g–i).
Living habitus and environment
The holotype of Scytalium herklotsi sp. nov. was photographed and collected by the ROV Deep Discoverer (Fig. 6) (see all photos associated with the registration code USNM 1550636 on the website “Search the Department of Invertebrate Zoology Collections” of the of the Smithsonian Institution, National Museum of Natural History (https://collections.nmnh.si.edu/search/iz/)). The fully expanded specimen (Fig. 6a) is white to deep red depending on the density of the coloured sclerites, dominant in the basal part of the autozooids including digitiform processes (Fig. 6b, insert) and the rachis peduncle limits (Fig. 6c), as well as the polyp-free dorsal track and mid to lower polyp leaves (Fig. 6e). It is worth mentioning that the autozooids, placed in a single row in each polyp leaf (see above), more or less alternatively expand in different directions (up–down, see Fig. 6b), giving the false impression of being arranged in different rows. According to the expedition report (Wagner et al. 2019, p. 98), the habitat observed during the EX1811-Dive07 included some boulders, but was mainly dominated by soft sediments, benthic fauna included at least 12 species of corals (mainly hydrocorals, antipatharians, chrysogorgiids, isidids, plexaurids, and ellisellids), sponges (hexactinelids and demosponges), and echinoderms, as well as some demersal fishes.
In situ photographs of Scytalium herklotsi sp. nov. Holotype (USNM 1550636). a Whole extended colony (lasers are 10 cm apart); b Detail of expanded autozooids, notice polyps oriented on opposite sides alternatively, and the distinct digitiform processes and tentacles (insert); c Detail of the expanded rachis-peduncle limit and lowest polyp leaves; d Precise moment of collection by the arm of the ROV Deep Discoverer; e Colony just after collection. Photos by NOAA Office of Ocean Exploration and Research (see all photos associated with the registration code USNM 1550636 on the website “Search the Department of Invertebrate Zoology Collections” of the Smithsonian Institution, National Museum of Natural History (https://collections.nmnh.si.edu/search/iz/)
Etymology
The name herklotsi is chosen in honour of Dutch zoologist Jan Adrianus Herklots (1820–1872), curator of invertebrates at the Rijksmuseum van Natuurlijke Historie in Leiden (1846–1872), in recognition of his contributions to the knowledge of sea pens and as the author who described the genus Scytalium and its type species, S. sarsii.
Geographical and depth distribution
At present, Scytalium herklotsi sp. nov. is only known from the Caribbean Sea (Puerto Rico) from 489 m depth. The new species is the only one known with certainty for the Atlantic Ocean; all other species in the genus have been reported for the Indian and Pacific Oceans.
Phylogenetic analyses
In our mtMutS+ND2+Cox1+28S hypothesis (Fig. 7), the multiloci sequence obtained for Scytalium herklotsi sp. nov. was reunited in a distinct and well-supported clade (Bst >100%, PP 1) with other sequences identified as Scytalium species from GenBank. The position of Scytalium herklotsi sp. nov. is basal, being the sister group of all other Scytalium sequences in a moderately supported clade (Bst >72%, PP 1). The genus Scytalium was the sister group of a sequence identified as Pennatula sp., a relatively well-supported clade (Bst >94%, PP 1). This last is a divergent sequence that deserves further research, as the core of the genus Pennatula (sequences close to Pennatula phosphorea Linnaeus, 1758) is quite distant but in the same Clade II. As mentioned above, although Clade II is well-supported, the basal resolution is poor. Well-supported clades can only be found at derived situations, as the clade Acanthoptillum-Renilla (Bst >98%, PP 1), or those groupings representing genera with more than a single species, such as Pennatula Linnaeus, 1758 (Bst >98%, PP 1), or Renilla Lamarck, 1816 (Bst >100%, PP 1). The genus Ptilella Gray, 1870, is well supported by both ML (Bst >99%) and BI (PP 1) methods, although the topology of both trees is slightly different (see Fig. 7, Supplemental Figure 1).
Bayesian analysis showing the phylogenetic relationships of Scytalium herklotsi sp. nov. with congeners and other genera and species of sea pens within Clade II (see Dolan et al. 2013, Kushida and Reimer 2018). The present hypothesis is based on the concatenated set of sequences of four markers: mtMutS+ND2+Cox1+28S. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Supporting values of nodes as Bootstrap (%)/Posterior probability. See Table 1 for complete list of species and GenBank accession numbers. The asterisk indicates a divergent sequence attributed to the genus Pennatula, but far from the true core of this genus (see “Phylogenetic analyses” section)
In a selected dataset considering only mtMutS, uncorrected p-distances indicated that the average genetic distance within Scytalium species (considering one sequence per supposed species) is 1.8% (varying depending on the pair of species from 0.0 to 2.8%). Scytalium herklotsi sp. nov. is 2.0%, 1.7%, and 1.7% distant from Scytalium sp. 1, Scytalium martensii, and Scytalium sp. 2, respectively. The divergent sequence attributed to Pennatula sp., sister group of the Scytalium spp., is 4.5% distant.
Discussion
According to Hickson (1916) and Williams (1995), on the most comprehensive and recent contributions discussing the diagnostic features to be used in the delimitation of Scytalium species, at least six nominal species are known. Among them, on the one hand, Scytalium tentaculatum and S. balssii are differentiated by the presence of a single digitiform process on the basal part of the polyp (named as calyx for some authors) (see Kölliker 1880, p. 10, Pl. VI, fig. 13, Hickson 1916, p. 205, Pl.VIII, figs. 55–56), both species differentiated by the amount of undeveloped polyp leaves proximally (see Hickson 1916, p. 205).
On the other hand, Scytalium sarsii and S. martensii are characterized by the scarce development of their polyp leaves and up to 30 (usually less) autozooids (see Herklots 1858, p. 14, fig. 6; Kölliker 1869–72, p. 574, Tb. IX, figs. 80–81; Thomson and Simpson, 1909, p. 284; Hickson 1916, p. 205). The former species has overlapping polyp leaves, without a naked ventral region (or very reduced) on the rachis, and the axis is quadrangular in cross section, while the latter has polyp leaves not overlapping, a naked ventral region on the rachis, and the axis is quadrangular with rounded angles.
Finally, Scytalium splendens differs from the previous species in being relatively stout with large polyp leaves and numerous autozooids (up to around 60) (Thomson and Henderson 1906, p. 116). A second species described by Thomson and Henderson, Scytalium veneris, is considered of uncertain validity by some authors (synonymized under the incorrect spelling “Scytalium sarsi” by Kükenthal and Broch 1911, p. 311; see Cordeiro et al. 2021). Hickson (1916, p. 205) considered this form related to S. splendens, but different because of the peculiar arrangement of the lower polyp leaves and the arrangement of siphonozooids, among other characteristics, although in the key to species a character is used that could be considered size(age?)-related. According to the original description, Scytalium veneris shows some particular features, such as the whip-like proximal polyp leaves simplified to a single autozooid, red sclerites on the aboral side of tentacles, and a different arrangement of siphonozooids bands on the ventral, lateral, and dorsal margins of the rachis (prorachidial margins, pararachidials, and metarachidial margins respectively, as named in some old literature) (see Thomson and Henderson 1906, pp. 115–118).
Waiting for a revision of the different type materials of the Scytalium species mentioned above, I will consider them all for the present discussion and comparison of characters with the new species found in the Caribbean (see also Table 2 for a more complete comparison of characters among all these species).
Scytalium herklotsi sp. nov. clearly differs from S. tentaculatum and S. balssii due to the presence of eight digitiform and spiculiferous processes on the distal part of the anthostele where anthocodia can retract, instead of the single digitiform process present in the Indian species.
The new species of Scytalium differs from S. sarsii and S. martensii by important differences in the development of the polyp leaves and colony, as well as in the number and arrangement of autozooids. In S. sarsii, 18–20 autozooids are reported to be (at least apparently) in two rows (see Herklots 1858: fig. 6), and siphonozooids are in two rows between polyp leaves, leaving latero-dorsal and dorsal areas polyp-free and colourless (without or with scarce sclerites). In contrast, apart from the development of polyp leaves and its, up to 35, relatively large autozooids per leaf, S. herklotsi sp. nov. shows a linear placement of autozooids, and a continuous longitudinal band of 3–6 rows of siphonozooids latero-dorsally, and an additional red dorsal track (due to the colour of the platelet sclerites) (see Fig. 2d). The type material of S. martensii is a slender and delicate colony of 49.3 cm in length, with 158 well-separated short polyp leaves. Different dimensions provided by Kölliker on polyp leaves and thickness of axis/rachis suggest that this number of polyp leaves is the total; and therefore, that number must be divided by two for our comparison, ~79 pairs of polyp leaves. Poly leaves are of approximately 3–3.5 mm in length and up to 13 relatively small autozooids of 0.45–0.54 mm length and 0.24–0.36 mm width. Siphonozooids are difficult to observe (0.16 mm in diameter) in an oblique row following the dorsal side of the polyp leaf. On the contrary, the type material of S. herklotsi sp. nov. is a large but delicate colony of 60 cm length, just about 10 cm longer than the holotype of S. martensii, but with just 37 overlapping and large polyp leaves of about 68 mm in length and up to 35 autozooids of up to 5 mm length and 1.4 mm width. The numerous siphonozooids (~0.18 mm in diameter) are easily visible as they conform distinctive bands of 3–6 rows between polyp leaves and as two longitudinal bands latero-dorally. Furthermore, none of these species has eight long digitiform processes on the basal part of the autozooids distally.
Scytalium herklotsi sp. nov. is closely related to the two last mentioned species, namely S. splendens and S. veneris, by sharing large and well-developed polyp leaves. The new Caribbean Atlantic species here proposed is close but differs from the Indian species by some characters that can perfectly be considered as species-discriminant. The new species shares with S. splendens the lack of whip-like proximal polyp leaves (present in S. veneris); however, the arrangement of the autozooids of S. herklotsi sp. nov. is more similar to that of S. veneris (see Thomson and Henderson 1906: pl. VIII, fig. 8) than to the congested ventral border of the polyps-leaves of S. splendens, where the autozooids are so numerous that they give the appearance of being placed in two or three rows (see Thomson and Henderson 1906: pl. VIII, fig. 5). Scytalium herklotsi sp. nov. is the only species reported so far with an axis rounded in cross section; all reports on the remaining species in the genus mention a quadrangular to quadrangular with rounded angles axis. The finding of an additional type of sclerite in the pharynx of S. herklotsi sp. nov. is considered here as a possible characteristic at the species level, although the presence or absence of these sclerites in other Scytalium species should be confirmed in future investigations.
Finally, although geographical and bathymetric comparisons must always be considered with caution since they represent a precise moment in the state of knowledge, Scytalium herklotsi sp. nov. is the only species in the genus known with certainty to be living in the Atlantic Ocean, and it is also the deepest record of a species of this genus (489 m in depth), while the distribution of the genus Scytalium was previously considered Indo-West Pacific, from shallow waters to the limits of the continental shelf (between 10 and 210 m in depth) (see Williams 1995, 2011; Imahara and Namikawa 2018).
Two additional aspects resulting from the descriptive part of Scytalium herklotsi sp. nov. are noteworthy: (1) the report for the first time in this genus of a second type of sclerite, those abundant minute ovals from the pharynx; and (2) the presence of developing autozooids on the proximal part of the ventral edge of the polyp leaves. These latter polyps have here been considered as developing autozooids due to the gradation in sizes observed between them, although it is noticeable that there is a great difference in size between the largest of these developing polyps and the first fully developed autozooid. It would be helpful if these two aspects could be verified in other species of Scytalium in order to increase our knowledge concerning the morphological variability of the usable taxonomic characters in this genus. Due to the lack of information in the published literature, these characteristics have not been used in the previous comparative discussion, but they have been included in the comparative table of species (Table 2).
According to Kushida and Reimer (2018), and García-Cárdenas et al. (2020), the family Virgulariidae is polyphyletic, the genus Scytalium being more related to some genera currently included in the family Pennatulidae, such as Pennatula or Ptilella (Dr. Xu, pers. comm.), than to other virgulariid genera. Indeed, the genus Virgularia Lamarck, 1816 (the type genus of the family), is placed in Clade I, as the sister group of the genus Pteroeides Herklots, 1858, while other sequenced virgulariid genera (such as Stylatula Verrill, 1864, and Acanthoptilum Kölliker, 1870 in Kölliker 1869-72) are in Clade II (see García-Cárdenas et al. 2020: figs. 2, S2). At present, the poor basal resolution of the lineages in Clade II obscures the possible delimitation of families; and therefore, the family assignment of some genera currently in Pennatuliidae or Virgulariidae should be considered tentative.
References
Balss H (1909) Über Pennatuliden des Münchener Museums. Zool Anz 34(113/14):423–431
Balss H (1910) Japanische Pennatuliden. Abh Math Phys Kl K Bayer Ak Wiss 1(10, suppl.):1–106
Bayer FM, Stefani J (1988) Primnoidae (Gorgonacea) de Nouvelle-Caledonie. Bull Mus Natl Hist Nat 10(A)3:449–476
Bayer FM, Grasshoff M, Verseveldt J (1983) Illustrated trilingual glossary of morphological and anatomical terms applied to Octocorallia. Brill/ Backhuys, Leiden
Braet F, Zanger R, Wisse E (1997) Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. J Microsc 186(1):84–87
Cordeiro R, McFadden C, van Ofwegen L, Williams G (2021) World list of octocorallia. Scytalium Herklots, 1858. World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=205981. Accessed 30 Mar 2021
Dolan E, Tyler PA, Yesson C, Rogers AD (2013) Phylogeny and systematics of deep-sea sea pens (Anthozoa: Octocorallia: Pennatulacea). Mol Phylogenet Evol 69(3):610–618. https://doi.org/10.1016/j.ympev.2013.07.018
France SC, Hoover LL (2002) DNA sequences of the mitochondrial COI gene have low levels of divergence among deep-sea octocorals (Cnidaria: Anthozoa). Hydrobiologia 471:149–155. https://doi.org/10.1023/A:1016517724749
García-Cárdenas FJ, Núñez-Flores M, López-González PJ (2020) Molecular phylogeny and divergence time estimates in pennatulaceans (Cnidaria: Octocorallia: Pennatulacea). Sci Mar 84:317–330. https://doi.org/10.3989/scimar.05067.28A
Gray JE (1860) Revision of the family Pennatulidae, with descriptions of some new species in the British Museum. Ann Mag Nat Hist Ser 35:20–25
Gray JE (1870) Catalogue of sea-pens or Pennatulariidae in the collection of the British Museum, London. https://doi.org/10.5962/bhl.title.11307
Haeckel E (1866) Generelle Morphologie der organismen. Georg Reimer. Berlin
Herklots JA (1858) Notices pour servir à l’étude des polypiers nageurs ou pennatulidés. Bijdr Dierkd 7:1–31
Hickson SJ (1916) The Pennatulacea of the Siboga Expedition, with a general survey of the order. Siboga Exped Monogr 14(77):1–265
Huelsenbeck JP, Ronquist F (2001) MrBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755. https://doi.org/10.1093/bioinformatics/17.8.754
Imahara Y, Namikawa H (2018) Preliminary report on the octocorals (Cnidaria: Anthozoa: Octocorallia) from the Ogasawara Islands. Mem Natl Mus Nat Sci Tokyo 52:65–94
Kölliker RA (1869-72) Anatomisch-Systematische Beschreibung der Alcyonararien. I. Die Pennatuliden. Abh Senckenb Naturforsch Ges 7–8:1–458
Kölliker RA (1880) Report on the Pennatulida dredged by H. M. S. Challenger during the years 1873-1876. Rep Sci Res Voy HMS Challenger 1873-76. Zoology 1(2):1–41
Kükenthal W (1915) Pennatularia. Das Tierreich. Verlag von R. Friedländer und Sohn, Berlin
Kükenthal W, Broch H (1911) Pennatulacea. Wiss Erg Deuts Tiefsee-Exped "Valdivia" 13(2):113–576
Kushida Y, Reimer JD (2018) Molecular phylogeny and diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea) with a focus on shallow water species of the northwestern Pacific Ocean. Mol Phylogenet Evol 131:233–244. https://doi.org/10.1016/j.ympev.2018.10.032
Lamarck JBPA (1816) Histoire naturelle des animaux sans vertèbres, Paris
Linnaeus C (1758) Systema naturae. Editio decima, reformata, Holmiae
López-González PJ (2020) A new calcaxonian genus and family for Trichogorgia utinomii Cordeiro, 2019 (Octocorallia, Alcyonacea): new records of a scleriteless gorgonian species from Antarctica. Mar Biodivers 50:96. https://doi.org/10.1007/s12526-020-01109-0
McFadden CS, Tullis ID, Hutchinson MB et al (2004) Variation in coding (NADH dehydrogenase subunits 2, 3, and 6) and noncoding intergenic spacer regions of the mitochondrial genome in Octocorallia (Cnidaria: Anthozoa). Mar Biotechnol 6:516–526. https://doi.org/10.1007/s10126-002-0102-1
Nation JL (1983) A new method using hexamethyldisilazane for preparation of soft insect tissues for Scanning Electron Microscopy. Stain Technol 58(6):347–351
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst Biol 67:901–904. https://doi.org/10.1093/sysbio/syy032
Richiardi S (1869) Monografia della famiglia dei Pennatularii. Arch Zool Anat Fisiol Turin (2)1:1–150
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12):1572–1574
Sánchez J, McFadden CS, France S, Lasker H (2003) Molecular phylogenetic analyses of shallow-water Caribbean octocorals. Mar Biol 142:975–987. https://doi.org/10.1007/s00227-003-1018-7
Shively S, Miller WR (2009) The use of HMDS (hexamethyldisilazana) to replace Critical Point Drying (CPD) in the preparation of tardigrades for SEM (Scanning Electron Microscope) imaging. Trans Kans Acad Sci 112(3/4):198–200. https://doi.org/10.1660/062.112.0407
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Thomson JA, Henderson WD (1906) An account of the alcyonarians collected by the Royal Indian Marine Survey Ship Investigator in the Indian Ocean. Part I. The alcyonarians of the deep sea. Trustees of the Indian Museum, Calcutta
Thomson JA, Simpson JJ (1909) An account of the alcyonarians collected by the Royal Indian Marine Survey Ship Investigator in the Indian Ocean. II. The alcyonarians of the littoral area. Trustees of the Indian Museum, Calcutta
Verrill AE (1864) List of the polyps and corals sent by the Museum of Comparative Zoology to other institutions in exchange, with annotations. Bull Mus Comp Zool Harvard College 1(3):29–60
Verrill AE (1865) Synopsis of the polyps and corals of the North Pacific Exploring Expedition, under Commodore C. Ringgold and Captain John Rogers, U.S.N., from 1853 to 1856. Collected by Dr. W. Stimpson, naturalist of the Expedition. With descriptions of some additional species from the west coast of North America. Proc Essex Inst 4:181–196
Verrill AE (1868-70) Notes on Radiata in the Museum of Yale College. No. 6. Review of the corals and polyps of the west coast of America. Trans Conn Acad Arts Sci 1: 377–558
Wagner D, Sowers D, Williams SM, Auscavitch S, Blaney D, Cromwell M (2019) EX-18-11 Expedition Report - Océano Profundo 2018: exploring deep-sea habitats off Puerto Rico and the U.S. Virgin Islands. NOAA, Silver Spring. https://doi.org/10.25923/wc2n-qg29
Williams GC (1995) Living genera of sea pens (Coelenterata: Octocorallia: Pennatulacea): illustrated key and synopses. Zool J Linnean Soc 113:93–140
Williams GC (1999) Index Pennatulacea: annotated bibliography and indexes of the Sea Pens (Coelenterata: Octocorallia) of the World 1469-1999. Proc Calif Acad Sci 51:19–103
Williams GC (2011) The global diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea). PLoS One 6:e22747. https://doi.org/10.1371/journal.pone.0022747
Acknowledgments
The author would like to express his gratitude to Stephen Cairns and the staff of the Smithsonian Institution for making the type material of the new Scytalium species here proposed available for study. I also express my gratitude to the cruise leader, D. Wagner, and participants on board the Océano Profundo 2018 cruise for the collection of this interesting sample. Thanks to Jeroen Goud (Naturalis Biodiversity Center, Leiden), who provided images and comments on the type material of Scytalium sarsii, the type species of the genus, as well as to Imahar Yukimitsu (Biological Institute on Kuroshio, Wakayama, Japan) for additional information on Scytalium specimens collected in Japanese waters. I would like to thank numerous colleagues and cruise leaders who have worked on the campaigns during which the material examined here was obtained: BIOROSS, BIOICE, ANT XXIII/8, SCOTIA cruises, and INDEMARES-Chica. On these cruises, our special thanks are addressed to Wolf Arntz, Josep-Maria Gili, Jim Drewery, Gudmundur Gudmundsson, Gudmundur Vidir, Jörundur Svavarsson, Stefano Schiaparelli, Annenina Lortz, Julian Gutt, Enrique Isla, Victor Díaz-del-Río, and José Luis Rueda. I also acknowledge financial support for a visit to the Sandgerdi Marine Centre (Iceland) under the EC-funded TMR BIOICE Large-Scale Facility Programme. I also acknowledge Dr. José Martín Garrido for his help in completing part of DNA amplifications. The study of the Antarctic specimens and the final conception of this paper were carried out under the project CTM2017- 83920-P (DIVERSICORAL) funded by the Spanish Ministry of Economy, Industry and Competitiveness. Mr Tony Krupa is thanked for reviewing the English version. The author would like to thank the two anonymous reviewers and the Editorial Office of MB for all their constructive comments and suggestions, which helped to improve the quality of an early version of the manuscript. Dr. Xu (Institute of Oceanology, Chinese Academy of Sciences) is also thanked for his comments on the polyphyly of the genera currently included in the family Virgulariidae, with special emphasis on the placement of the genus Scytalium.
Funding
The collection of some of the specimens here studied was carried out thanks to the Spanish Projects POL2006-06399/CGL (Polarstern ANT XXIII/8 - CLIMANT), and LIFE07/NAT/E/000732 LIFE+INDEMARES. This research is supported by the project CTM2017-83920-P (DIVERSICORAL), Spanish Ministry of Economy, Industry and Competitiveness.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for animal testing, animal care and use of animals were followed by the author.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the author (or responsible researchers of the different research programs) from the competent authorities and are mentioned in the acknowledgements.
Data availability
The data generated and analysed during this study are deposited in public repositories.
Additional information
Communicated by B. W. Hoeksema
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is registered in ZooBank under http://zoobank.org/5982870F-635A-4630-8DB5-C1CF29CE93D4
Supplementary Information
ESM 1
SMFig. 1 ML analysis showing the phylogenetic relationships of Scytalium herklotsi sp. nov. with congeners and other genera and species of sea pens within Clade II (see Dolan et al. 2013, Kushida and Reimer 2018). The present hypothesis is based on the concatenated set of sequences of four markers: mtMutS+ND2+Cox1+28S. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. Supporting values (Bootstrap) are in percentages. See Table 1 for complete list of species and GenBank accession numbers. The asterisk indicates a divergent sequence attributed to the genus Pennatula, but far from the true core of this genus (see Phylogenetic analyses section). (PNG 747 kb)
Rights and permissions
About this article
Cite this article
López-González, P.J. Scytalium herklotsi sp. nov. (Anthozoa, Octocorallia, Pennatulacea), the first Atlantic species in the genus Scytalium Herklots, 1858. Mar. Biodivers. 51, 62 (2021). https://doi.org/10.1007/s12526-021-01200-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-021-01200-0