Abstract
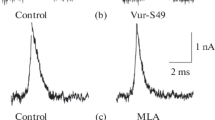
The main goal of the study was to analyze the relationship between the signaling pathways regulating acetylcholine (ACh) quantal release in the peripheral synapse that are triggered by the activation of vanilloid (TRPV1) and purine receptors. In electrophysiological experiments carried out on the neuromuscular synapse of the mouse levator auris longus muscle, it was found that the frequency of miniature end-plate potentials (mEPPs) and the quantal content of end-plate potentials (EPPs) decrease in the presence of the TRPV1 receptor agonist capsaicin. This effect was completely reversed by SB 366791, a specific competitive TRPV1 receptor antagonist. ATP, like capsaicin, decreased the frequency of mEPPs and the EPP quantal content. In the presence of SB 366791, the inhibitory effect of ATP on ACh secretion realized its full. At the same time, in the presence of TRPV1 channel activation by capsaicin, the effect of ATP on both spontaneous and evoked ACh release was absent. It was suggested that the action mechanisms of ATP and capsaicin are underpinned by a change in Ca2+ entry into the nerve ending. To test this hypothesis, experiments were carried out to assess the changes in the presynaptic calcium level (Ca2+ transient) using a fluorescent calcium dye upon nerve stimulation. The amplitude of the Ca2+ transient did not change by either ATP application or capsaicin addition. Thus, in the neuromuscular synapse of mammals, along with the purinergic pathway of ACh secretion regulation, there is also a mechanism of neurosecretion modulation mediated by the activation of TRPV1 channels. The triggering of these mechanisms leads to the suppression of the processes of both spontaneous and evoked ACh quantal release from the motor nerve endings. It was demonstrated that both regulatory pathways are not accompanied by a change in the Ca2+ transient, but share a common link in ACh quantal release regulation.






Similar content being viewed by others
REFERENCES
Fagerlund MJ, Eriksson LI (2009) Current concepts in neuromuscular transmission. Br J Anaesth 103:108–114. https://doi.org/10.1093/bja/aep150
Petrov KA, Nikolsky EE, Masson P (2018) Autoregulation of acetylcholine release and micro-pharmacodynamic mechanisms at neuromuscular junction: Selective acetylcholinesterase inhibitors for therapy of myasthenic syndromes. Front Pharmacol 9:1–8. https://doi.org/10.3389/fphar.2018.00766
Kilbinger H (1996) Modulation of acetylcholine release by nitric oxide. Prog Brain Res 109:219–224. https://doi.org/10.1016/s0079-6123(08)62105-6
Datar P, Srivastava S, Coutinho E, Govil G (2005) Substance P: Structure, Function, and Therapeutics. Curr Top Med Chem 4:75–103. https://doi.org/10.2174/1568026043451636
Pinard A, Lévesque S, Vallée J, Robitaille R (2003) Glutamatergic modulation of synaptic plasticity at a PNS vertebrate cholinergic synapse. Eur J Neurosci 18:3241–3250. https://doi.org/10.1111/j.1460-9568.2003.03028.x
Malomouzh AI, Nikolsky EE, Lieberman EM, Sherman JA, Lubischer JL, Grossfeld RM, Urazaev AK (2005) Effect of N-acetylaspartylglutamate (NAAG) on non-quantal and spontaneous quantal release of acetylcholine at the neuromuscular synapse of rat. J Neurochem 94:257–267. https://doi.org/10.1111/j.1471-4159.2005.03194.x
Ribeiro JA, Cunha RA, Correia-de-Sa P, Sebastiao AM (1996) Purinergic regulation of acetylcholine release. Prog Brain Res 109:231–241. https://doi.org/10.1016/s0079-6123(08)62107-x
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797. https://doi.org/10.1152/physrev.00043.2006
Surprenant A, North RA (2009) Signaling at purinergic P2X receptors. Annu Rev Physiol 71:333–359. https://doi.org/10.1146/annurev.physiol.70.113006.100630
Grishin S, Shakirzyanova A, Giniatullin A, Afzalov R, Giniatullin R (2005) Mechanisms of ATP action on motor nerve terminals at the frog neuromuscular junction. Eur J Neurosci 21:1271–1279. https://doi.org/10.1111/j.1460-9568.2005.03976.x
Ginsborg BL, Hirst GDS (1972) The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol 224:629–645. https://doi.org/10.1113/jphysiol.1972.sp009916
Khaziev EF, Samigullin DV, Tsentsevitsky AN, Bukharaeva EA, Nikolsky EE (2018) ATP Reduces the Entry of Calcium Ions into the Nerve Ending by Blocking L-type Calcium Channels. Acta Naturae 10:93–96. https://doi.org/10.32607/20758251-2018-10-2-93-96
Tominaga M, Wada M, Masu M (2001) Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A 98:6951–695. https://doi.org/10.1073/pnas.111025298
Morales-Lázaro Sara L., Simon Sidney A, Rosenbaum T (2013) The role of endogenous molecules in modulating pain through TRPV1. J Physiol 591:3109–3121. https://doi.org/10.1113/jphysiol.201
Lishko PV., Procko E, Jin X, Phelps CB, Gaudet R (2007) The Ankyrin Repeats of TRPV1 Bind Multiple Ligands and Modulate Channel Sensitivity. Neuron 54:905–918. https://doi.org/10.1016/j.neuron.2007.05.027
Fernández-Carvajal A, Fernández-Ballester G, Devesa I, González-Ros JM, Ferrer-Montiel A (2011) New strategies to develop novel pain therapies: Addressing thermoreceptors from different points of view. Pharmaceuticals 5:16–48. https://doi.org/10.3390/ph5010016
Thyagarajan B, Krivitskaya N, Potian JG, Hognason K, Garcia CC, McArdle JJ (2009) Capsaicin protects mouse neuromuscular junctions from the neuroparalytic effects of botulinum neurotoxin. J Pharmacol Exp Ther 331:361–371. https://doi.org/10.1124/jpet.109.156901
Thyagarajan B, Potian JG, Baskaran P, McArdle JJ (2014) Capsaicin modulates acetylcholine release at the myoneural junction. Eur J Pharmacol 744:211–219. https://doi.org/10.1016/j.ejphar.2014.09.044
Martin AR (1976) The effect of membrane capacitance on non-linear summation of synaptic potentials. J Theor Biol 59:179–187. https://doi.org/10.1016/S0022-5193(76)80031-8
Samigullin DV., Khaziev EF, Zhilyakov NV, Sudakov IA, Bukharaeva EA, Nikolsky EE (2017) Calcium Transient Registration in Response to Single Stimulation and During Train of Pulses in Mouse Neuromuscular Junction. Bionanoscience 7:162–166. https://doi.org/10.1007/s12668-016-0318-6
De Lorenzo S, Veggetti M, Muchnik S, Losavio A (2006) Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience 142:71–85. https://doi.org/10.1016/j.neuroscience.2006.05.062
Galkin AV., Giniatullin RA, Mukhtarov MR, Švandová I, Grishin SN, Vyskočil F (2001) ATP but not adenosine inhibits nonquantal acetylcholine release at the mouse neuromuscular junction. Eur J Neurosci 13:2047–2053. https://doi.org/10.1046/j.0953-816X.2001.01582.x
Augustine GJ (2001) How does calcium trigger neurotransmitter release? Curr Opin Neurobiol 11:320–326. https://doi.org/10.1016/S0959-4388(00)00214-2
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389:816–824. https://doi.org/10.1038/39807
Samigullin DV., Zhilyakov NV., Khaziev EF, Bukharaeva EA, Nikolsky EE (2018) Calcium Transient and Quantal Release in Mouse Neuromuscular Junction Under Extracellular Calcium Concentration Change. Bionanoscience 8:984–987. https://doi.org/10.1007/s12668-018-0558-8
Ziganshin AU, Khairullin AE, Hoyle CHV, Grishin SN (2020) Modulatory roles of ATP and adenosine in cholinergic neuromuscular transmission. Int J Mol Sci 21:1–15. https://doi.org/10.3390/ijms21176423
Voss AA (2009) Extracellular ATP inhibits chloride channels in mature mammalian skeletal muscle by activating P2Y1 receptors. J Physiol 587:5739–5752. https://doi.org/10.1113/jphysiol.2009.179275
Gaydukov AE, Melnikova SN, Balezina OP (2009) Facilitation of acetylcholine secretion in mouse motor synapses caused by calcium release from depots upon activation of L-type calcium channels. Bull Exp Biol Med 148:163–166. https://doi.org/10.1007/s10517-009-0678-9
Khuzakhmetova VF, Samigullin DV, Bukharaeva EA (2014) The role of presynaptic ryanodine receptors in regulation of the kinetics of the acetylcholine quantal release in the mouse neuromuscular junction. Biochem Suppl Ser A Membr Cell Biol 8:144–152. https://doi.org/10.1134/S199074781305005X
Filippov AK, Brown DA, Barnard EA (2000) The P2Y1 receptor closes the N-type Ca2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. Br J Pharmacol 129:1063–1066. https://doi.org/10.1038/sj.bjp.0703185
Marshall ICB, Owen DE, Cripps TV, Davis JB, McNulty S, Smart D (2003) Activation of vanilloid receptor 1 by resiniferatoxin mobilizes calcium from inositol 1,4,5-trisphosphate-sensitive stores. Br J Pharmacol 138:172–176. https://doi.org/10.1038/sj.bjp.0705003
Tarasova EO, Gaydukov AE, Balezina OP (2018) Calcineurin and Its Role in Synaptic Transmission. Biochem 83:674–689. https://doi.org/10.1134/S0006297918060056
Ando K, Kudo Y, Aoyagi K, Ishikawa R, Igarashi M, Takahashi M (2013) Calmodulin-dependent regulation of neurotransmitter release differs in subsets of neuronal cells. Brain Res 1535:1–13. https://doi.org/10.1016/j.brainres.2013.08.018
Zhang Z, Nguyen KT, Barrett EF, David G (2010) Vesicular ATPase Inserted into the Plasma Membrane of Motor Terminals by Exocytosis Alkalinizes Cytosolic pH and Facilitates Endocytosis. Neuron 68:1097–1108. https://doi.org/10.1016/j.neuron.2010.11.035
Rodrigues AZC, Wang ZM, Messi ML, Delbono O (2019) Sympathomimetics regulate neuromuscular junction transmission through TRPV1, P/Q- and N-type Ca 2+ channels. Mol Cell Neurosci 95:59–70. https://doi.org/10.1016/j.mcn.2019.01.007
Funding
This study was supported by the Russian Foundation for Basic Research, grant no. 19-04-00490 (experimental part) and a budget funding within the Government assignment to Kazan Institute of Biochemistry and Biophysics, Kazan Scientific Center, Russian Academy of Sciences (processing of fluorescent images).
Author information
Authors and Affiliations
Contributions
Basic idea and planning of the experiments (A.Y.A. and D.V.S.); data collection (A.Y.A. and N.V.Z.); manuscript writing and editing (A.Y.A., A.I.M. and D.V.S.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare neither evident nor potential conflict of interest in relation to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2021, Vol. 107, Nos. 4–5, pp. 647–660https://doi.org/10.31857/S0869813921040038.
Rights and permissions
About this article
Cite this article
Arkhipov, A.Y., Zhilyakov, N.V., Malomouzh, A.I. et al. Interaction between the Mechanisms of Suppression of Acetylcholine Quantal Secretion upon Activation of Vanilloid (TRPV1) and Purine Receptors in the Mouse Neuromuscular Synapse. J Evol Biochem Phys 57, 709–719 (2021). https://doi.org/10.1134/S0022093021030182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021030182