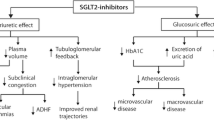
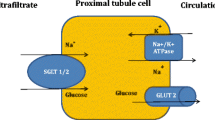
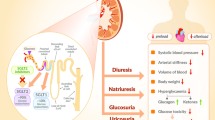
Abstract
Approximately 5 million individuals in the US are living with congestive heart failure (CHF), with 650,000 new cases being diagnosed every year. CHF has a multifactorial etiology, ranging from coronary artery disease, hypertension, valvular abnormalities and diabetes mellitus. Currently, guidelines by the American College of Cardiology advocate the use of angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, β-blockers, diuretics, aldosterone antagonists, and inotropes for the medical management of heart failure. The sodium glucose cotransporter 2 (SGLT2) inhibitors are a class of drug that have been widely used in the management of type 2 diabetes mellitus that work by inhibiting the reabsorption of glucose in the proximal convoluted tubule. Since the EMPA-REG OUTCOME trial, several studies have demonstrated the benefits of SGLT2 inhibitors in reducing cardiovascular risk related to heart failure. While the cardiovascular benefits could be explained by their ability to reduce weight, improve glycemic index and lower blood pressure, several recent trials have suggested that SGLT2 inhibitors exhibit pleiotropic effects that underlie their cardioprotective properties. These findings have led to an expansion in preclinical and clinical research aiming to understand the mechanisms by which SGLT2 inhibitors improve heart failure outcomes.




Similar content being viewed by others
References
Yancy C, Jessup M, Bozkurt B, Butler J, Casey D, Drazner M, et al. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147–239.
Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241(19):2035–8.
MacDonald M, Petrie M, Varyani F, Ostergren J, Michelson E, Young J, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29(11):1377–85.
Erqou S, Lee C, Suffoletto M, Echouffo-Tcheugui J, de Boer R, van Melle J, et al. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. Eur J Heart Fail. 2013;15(2):185–93.
Zinman B, Wanner C, Lachin J, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Neal B, Perkovic V, Mahaffey K, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott S, Raz I, Bonaca M, Mosenzon O, Kato E, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Bell RM, Yellon DM. SGLT2 inhibitors: hypotheses on the mechanism of cardiovascular protection. Lancet Diabetes Endocrinol. 2018;6(6):435–7.
Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Barsotti E, Clerico A, Muscelli E. Renal handling of ketones in response to sodium–glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2017;40(6):771–6.
Scholtes RA, Muskiet MHA, van Baar MJB, Hesp AC, Greasley PJ, Karlsson C, Hammarstedt A, Arya N, van Raalte DH, Heerspink HJL. Natriuretic Effect of two weeks of dapagliflozin treatment in patients with type 2 diabetes and preserved kidney function during standardized sodium intake: results of the DAPASALT trial. Diabetes Care. 2021;44(2):440–7.
Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab. 2018;20(3):479–87.
Cai X, Yang W, Gao X, Chen Y, Zhou L, Zhang S, Han X, Ji L. The association between the dosage of SGLT2 Inhibitor and weight reduction in type 2 diabetes patients: a meta-analysis. Obesity (Silver Spring). 2018;26(1):70–80.
Shah R, Gayat E, Januzzi JL Jr, Sato N, Cohen-Solal A, diSomma S, Fairman E, Harjola VP, Ishihara S, Lassus J, Maggioni A, Metra M, Mueller C, Mueller T, Parenica J, Pascual-Figal D, Peacock WF, Spinar J, van Kimmenade R, Mebazaa A, GREAT (Global Research on Acute Conditions Team) Network. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63(8):778–85.
Zamora E, Díez-López C, Lupón J, de Antonio M, Domingo M, Santesmases J, Troya MI, Díez-Quevedo C, Altimir S, Bayes-Genis A. Weight loss in obese patients with heart failure. J Am Heart Assoc. 2016;5(3):e002468.
McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM, DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Despa S, Islam M, Weber C, Pogwizd S, Bers D. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation. 2002;105(21):2543–8.
Pieske B, Maier LS, Piacentino V 3rd, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106(4):447–53.
Kim C, Fan T, Kelly P, Himura Y, Delehanty J, Hang C, et al. Isoform-specific regulation of myocardial Na, K-ATPase alpha-subunit in congestive heart failure. Role of norepinephrine. Circulation. 1994;89(1):313–20.
Dixon I, Hata T, Dhalla N. Sarcolemmal Na(+)-K(+)-ATPase activity in congestive heart failure due to myocardial infarction. Am J Physiol Cell Physiol. 1992;262(3):C664–71.
Lou Q, Janardhan A, Efimov IR. Remodeling of calcium handling in human heart failure. Adv Exp Med Biol. 2012;740:1145–74. https://doi.org/10.1007/978-94-007-2888-2_52.
Li J, Wu N, Dai W, et al. Association of serum calcium and heart failure with preserved ejection fraction in patients with type 2 diabetes. Cardivasc Diabetol. 2016;15(1):140. https://doi.org/10.1186/s12933-016-0458-6.
Liu T, Takimoto E, Dimaano VL, et al. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ Res. 2014;115(1):44–54. https://doi.org/10.1161/CIRCRESAHA.115.303062.
Baartscheer A, Schumacher C, Wüst R, Fiolet J, Stienen G, Coronel R, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2016;60(3):568–73.
Van Steenbergen A, Balteau M, Ginion A, Ferté L, Battault S, Ravenstein CM, Balligand JL, Daskalopoulos EP, Gilon P, Despa F, Despa S, Vanoverschelde JL, Horman S, Koepsell H, Berry G, Hue L, Bertrand L, Beauloye C. Sodium-myoinositol cotransporter-1, SMIT1, mediates the production of reactive oxygen species induced by hyperglycemia in the heart. Sci Rep. 2017;7:41166.
Trum M, Riechel J, Lebek S, Pabel S, Sossalla ST, Hirt S, Arzt M, Maier LS, Wagner S. Empagliflozin inhibits Na+/H+ exchanger activity in human atrial cardiomyocytes. ESC Heart Fail. 2020;7(6):4429–37.
Li C, Zhang J, Xue M, Li X, Han F, Liu X, Xu L, Lu Y, Cheng Y, Li T, Yu X, Sun B, Chen L. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18(1):15.
Balteau M, Tajeddine N, de Meester C, Ginion A, Des Rosiers C, Brady NR, Sommereyns C, Horman S, Vanoverschelde JL, Gailly P, Hue L, Bertrand L, Beauloye C. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc Res. 2011;92:237–46.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70.
Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, et al. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK- dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–33.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–804.
Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84(1):111–8.
Zhou L, Cryan EV, D’Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem. 2003;90(2):339–46.
Liang Y, Arakawa K, Ueta K, Matsushita Y, Kuriyama C, Martin T, Du F, Liu Y, Xu J, Conway B, Conway J, Polidori D, Ways K, Demarest K. Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS ONE. 2012;7(2):e30555.
Baartscheer A, Schumacher CA, van Borren MM, Belterman CN, Coronel R, Fiolet JW. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res. 2003;57:1015–24.
Packer M. Activation and inhibition of sodium–hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017;136(16):1548–59.
Despa S. Myocytes [Na+]I dysregulation in heart failure and diabetic cardiomyopathy. Front Physiol. 2018;9:1303.
Fliegel L. Regulation of the Na+/H+ exchanger in the healthy and diseased myocardium. Expert Opin Ther Targets. 2009;13:55–68.
Kili’c A, Huang CX, Rajapurohitam V, Madwed JB, Karmazyn M. Early and transient sodium-hydrogen exchanger isoform 1 inhibition attenuates subsequent cardiac hypertrophy and heart failure following coronary artery ligation. J Pharmacol Exp Ther. 2014;351:492–9.
Darmellah A, Baetz D, Prunier F, Tamareille S, Rücker-Martin C, Feuvray D. Enhanced activity of the myocardial NaC/HC exchanger contributes to left ventricular hypertrophy in the Goto-Kakizaki rat model of type 2 diabetes: critical role of Akt. Diabetologia. 2007;50:1335–44.
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of NaC/HC exchanger, lowering of cytosolic NaC and vasodilation. Diabetologia. 2018;61:722–6.
Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell. 1989;56:271–80.
Cingolani HE, Ennis IL. Sodium-hydrogen exchanger, cardiac overload, and myocardial hypertrophy. Circulation. 2007;115(9):1090–100.
Wakabayashi S, Hisamitsu T, Nakamura TY. Regulation of the cardiac Na+/H+ exchanger in health and disease. J Mol Cell Cardiol. 2013;61:68–76.
Odunewu-Aderibigbe A, Fliegel L. The Na+/H+ exchanger and pH regulation in the heart. Int Union Biochem Mol Biol. 2014;66:679–85.
Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J. The Na+/H antiporter cytoplasmic domain mediates growth factor signals and controls “H+-sensing.” Proc Natl Acad Sci USA. 1992;89:2424–8.
Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol. 2006;187:159–67.
Moor A, Gan XT, Karmazyn M, Fliegel L. Activation of Na1/H1 exchanger-directed protein kinases in the ischemic and ischemic reperfused rat myocardium. J Biol Chem. 2001;276:16113–22.
Karki P, Fliegel L. Overexpression of the NHE1 isoform of the Na(1)/H (1) exchanger causes elevated apoptosis in isolated cardiomyocytes after hypoxia/reoxygenation challenge. Mol Cell Biochem. 2010;338:47–57.
Medina AJ, Pinilla OA, Portiansky EL, Caldiz CI, Ennis IL. Silencing of the Na+/H+ exchanger 1(NHE-1) prevents cardiac structural and functional remodeling induced by angiotensin II. Exp Mol Pathol. 2019;107:9.
Mraiche F, Oka T, Gan XT, Karmazyn M, Fliegel L. Activated NHE1 is required to induce early cardiac hypertrophy in mice. Basic Res Cardiol. 2011;106:603–16.
Garciarena CD, Caldiz CI, Correa MV, Schinella GR, Mosca SM, de Cingolani CGE, Cingolani HE, Ennis IL. Na+/H+ exchanger-1 inhibitors decrease myocardialsuperoxide production via direct mitochondrial action. J Appl Physiol. 2008;105(6):1706–13.
Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302(2):148–58.
Ennis IL, Escudero EM, Console GM, Camihort G, Dumm CG, Seidler RW, de Hurtado MCC, Cingolani HE. Regression of isoproterenol -induced cardiac hypertrophy by Na+/H+ exchanger inhibition. Hypertension. 2003;41(6):1324–9.
Ennis IL, Garciarena CD, Escudero EM, Perez NG, Dulce RA, de Hurtadoand MCC, Cingolani HE. Normalization of the calcineurin pathway underlies the regression of hypertensive hypertrophy induced by Na+/H+ exchanger-1 (NHE-1) inhibition. Can J Physiol Pharmacol. 2007;85(3–4):301–10.
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “Thrifty Substrate” hypothesis. Diabetes Care. 2016;39(7):1108–14.
Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19(2):59–113.
Kuhlbrandt W. Structure and mechanisms of F-type ATP synthases. Annu Rev Biochem. 2019;88:21.1-21.35.
Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133(8):698–705.
Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, et al. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation. 2016;133(8):706–16.
Bayeva M, Sawicki KT, Ardehali H. Taking diabetes to heart–deregulation of myocardial lipid metabolism in diabetic cardiomyopathy. J Am Heart Assoc. 2013;2(6):e000433.
Ferrannini E, Muscelli E, Frascerra S, Frascerra S, Baldi S, Mari A, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508.
Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J. 2011;38(5):533–8.
Bratusch-Marrain P, Waldhausl W, Grubeck-Loebenstein B, Korn A, Vierhapper H, Nowotny P. The role of “diabetogenic” hormones on carbohydrate and lipid metabolism following oral glucose loading in insulin dependent diabetics: effects of acute hormone administration. Diabetologia. 1981;21(4):387–93.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–4.
Dutka M, Bobiński R, Ulman-Włodarz I, Hajduga M, Bujok J, Pająk C, Ćwiertnia M. Various aspects of inflammation in heart failure. Heart Fail Rev. 2020;25(3):537–48.
Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50–9. https://doi.org/10.15420/ecr.2018.33.1.
Kim SR, Lee SG, Kim SH, et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31(2):119–32.
Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med. 2017;104:298–310.
Nakatsu Y, Kokubo H, Bumdelger B, Yoshizumi M, Yamamotoya T, Matsunaga Y, Ueda K, Inoue Y, Inoue MK, Fujishiro M, Kushiyama A, Ono H, Sakoda H, Asano T. The SGLT2 inhibitor luseogliflozin rapidly normalizes aortic mRNA levels of inflammation-related but not lipid-metabolism-related genes and suppresses atherosclerosis in diabetic ApoE KO mice. Int J Mol Sci. 2017;18(8):1704.
Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, Choi SH, Jang HC, Lee HS, Park KS, Kim YB, Lim S. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia. 2017;60(2):364–76.
Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, Matsuzawa Y, Tomiyama H, Yamaoka-Tojo M, Yoshida H, Sato Y, Ikehara Y, Ueda S, Higashi Y, Node K, EMBLEM Investigators. Effect of empagliflozin on endothelial function in patients with type 2 diabetes and cardiovascular disease: results from the multicenter, randomized, placebo-controlled. Double-Blind EMBLEM Trial. Diabetes Care. 2019;42(10):e159–61.
Abazid RM, Smettei OA, Kattea MO, Sayed S, Saqqah H, Widyan AM, Opolski MP. Relation between epicardial fat and subclinical atherosclerosis in asymptomatic individuals. J Thorac Imaging. 2017;32(6):378–82.
Asghar A, Sheikh N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017;315:18–26.
Packer M. Do sodium-glucose co-transporter-2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis. Diabetes Obes Metab. 2018;20(6):1361–6.
Bailey CJ, Iqbal N, T’joen C, List JF. Dapagliflozin monotherapy in drug-naïve patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab. 2012;14(10):951–9.
Garvey WT, Van Gaal L, Leiter LA, Vijapurkar U, List J, Cuddihy R, Ren J, Davies MJ. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;85:32–7.
Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol. 2017;16(1):32.
Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12.
Sciarretta S, Yee D, Nagarajan N, et al. Trehalose-induced activation of autophagy improves cardiac remodeling after myocardial infarction. J Am Coll Cardiol. 2018;71(18):1999–2010.
Packer M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020;4:618–28.
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J. Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol. 2018;15:335–46.
Swe MT, Thongnak L, Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Lungkaphin A. Dapagliflozin not only improves hepatic injury and pancreatic endoplasmic reticulum stress, but also induces hepatic gluconeogenic enzymes expression in obese rats. Clin Sci (Lond). 2019;133(23):2415–30.
Bessho R, Takiyama Y, Takiyama T, Kitsunai H, Takeda Y, Sakagami H, Ota T. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. 2019;9(1):14754.
LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42(1):77–96.
Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. 2020;19(1):124.
Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, Chen L. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: A meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018;20(2):458–62.
Davies MJ, Trujillo A, Vijapurkar U, Damaraju CV, Meininger G. Effect of canagliflozin on serum uric acid in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17(4):426–9.
Li Z, Shen Y, Chen Y, Zhang G, Cheng J, Wang W. High uric acid inhibits cardiomyocyte viability through the ERK/P38 pathway via oxidative stress. Cell Physiol Biochem. 2018;45(3):1156–64.
Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, Teoh H, Leiter LA, Zinman B, Jüni P, Zuo F, Mistry N, Thorpe KE, Goldenberg RM, Yan AT, Connelly KA, Verma S. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2020;141(8):704–7.
Aberle J, Menzen M, Schmid SM, Terkamp C, Jaeckel E, Rohwedder K, Scheerer MF, Xu J, Tang W, Birkenfeld AL. Dapagliflozin effects on haematocrit, red blood cell count and reticulocytes in insulin-treated patients with type 2 diabetes. Sci Rep. 2020;10(1):22396.
Chiba Y, Yamada T, Tsukita S, et al. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS ONE. 2016;11(3):e0150756.
Herat LY, Magno AL, Rudnicka C, Hricova J, Carnagarin R, Ward NC, Arcambal A, Kiuchi MG, Head GA, Schlaich MP, Matthews VB. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci. 2020;5(2):169–79.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this review.
Conflict of Interests
Utkarsh Ojha, Lenisse Reyes, Florence Eyenga, Diane Oumbe, Justyna Watkowska and Henock Saint-Jacques have no conflicts of interest to declare.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the literature review and drafting or revising of the manuscript, gave final approval of the version to be published, and agree to be accountable for all aspects of this work.
Rights and permissions
About this article
Cite this article
Ojha, U., Reyes, L., Eyenga, F. et al. Diabetes, Heart Failure and Beyond: Elucidating the Cardioprotective Mechanisms of Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors. Am J Cardiovasc Drugs 22, 35–46 (2022). https://doi.org/10.1007/s40256-021-00486-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00486-6