Abstract
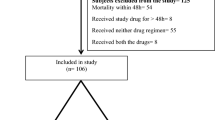
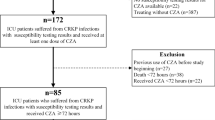
Infections by carbapenem-resistant Klebsiella pneumoniae (CRKp) are an increasing global threat with limited therapeutic options. Our objective was to evaluate clinical and microbiological outcomes of patients treated with amikacin for CRKp infections. We did a retrospective cohort of patients > 18 years old, with CRKp infections treated with amikacin in two tertiary care hospitals in Porto Alegre, Brazil. The impact of clinical factors, antibiotic treatment, and amikacin minimum inhibitory concentration (MIC) on patients’ 30-day mortality was assessed. Microbiological clearance and nephrotoxicity (assessed by RIFLE score) were evaluated as secondary outcomes. A Cox regression analysis was done for mortality. We included 84 patients for analysis. Twenty-nine (34.5%) patients died in 30 days. Amikacin MIC values ranged from 0.125 to 8 μg/mL and did not influence on mortality, regardless of the prescribed dose of this antibiotic (P = 0.24). Bacterial clearance occurred in 17 (58.6%) of 29 patients who collected subsequent cultures. Two (16.6%) of the 12 persistently positive cultures changed the amikacin susceptibility profile from susceptible to intermediate. Twenty-nine (37.2%) patients developed acute kidney injury (AKI): risk 13, injury 11, and failure 5. Risk factors for AKI were higher baseline eGFR (P < 0.01) and combination therapy with colistin (P = 0.02). Comparing patients who received combination with colistin vs polymyxin B, AKI occurred in 60.0% vs 20.6%, respectively, P < 0.01. Fifteen of the 16 (16.6%) patients who developed renal injury or failure were receiving colistin. In conclusion, amikacin was an effective treatment for CRKp infections. Within susceptible range, amikacin MIC values did not influence on clinical outcomes. Combination therapy of amikacin and colistin was highly nephrotoxic and should be used with caution.

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Bassetti M, Peghin M, Pecori D (2016) The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis 29:583–594
Gutierrez BG, Salamanca E, Cueto M, Hsueh P, Viale P, Pardo JRP (2017) Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 17:726–734
Medeiros GS, Rigatto MH, Falci DR, Zavascki AP (2019) Combination therapy with polymyxin B for carbapenemase-producing Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents 53(2):152–157
Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A et al (2012) Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clinical infect Dis: Off Publ Infect Dis Soc Am 55:943–950
Neuner EA, Gallagher JC (2016) Pharmacodynamic and pharmacokinetic considerations in the treatment of critically III patients infected with carbapenem-resistant Enterobacteriaceae. Virulence 4:440–452. https://doi.org/10.1080/21505594.2016.1221021
Zavascki AP, Klee BO, Bulitta JB (2017) Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther 15(6):519–526
Girlich D, Poirel L, Nordmann P (2012) Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J Clin Microbiol 50(2):477–479
Weiss D, Engelmanna I, Brauna SD, Moneckea S, Ehrichta R (2017) A multiplex real-time PCR for the direct, fast, economic and simultaneous detection of the carbapenemase genes blaKPC, blaNDM, blaVIM and blaOXA48. J Microbiol Methods 142:20–26
Clinical and Laboratory Standards Institute (2018) Performance standards antimicrobial susceptibility testing; twenty-six informational supplement M100S 2018. CLSI, Wayne
Bellomo R, Ronco C, Kellum JA, Metha RL, Palevsky P (2004) Acute dialysis quality initiative workgroup. Acute renal failure- definition, outcome measures, animal models, fluid therapy and information technology needs: the Second international Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Charlson ME, Pompei P, Ales KL et al (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Gilbert D, Chambers HF, Eliopoulus GM et al (2019) In: Sanford Guide to Antimicrobial Therapy, 490 Edition, ISBN 9788527736084
Shields RK, Clancy CJ, Press EG et al (2016) Aminoglycosides for treatment of bacteremia due to carbapenem-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 60(5):3187–3192. https://doi.org/10.1128/AAC.02638-15
Ong LZ, Tambyah PA, Lum LH et al (2016) Aminoglycoside-associated acute kidney injury in elderly patients with and without shock. J Antimicrob Chemother 71(11):3250–3257
De Oliveira MS, de Assis DB, Freire MP et al (2015) Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 21:179.e1-179.e7
Paquette F, Jean AB, Brunette V, Ammann H, Lavergne V, Pichette V et al (2015) Acute kidney injury and renal recovery with the use of aminoglycosides: a large retrospective study. Nephron 131:153–160
Swan SK (1997) Aminoglycoside nephrotoxicity. Semin Nephrol 17(1):27–33
Nation RL, Velkov T, Li J (2014) Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clinical Infect Dis: Off Publ Infect Dis Soc Am 59:88–94
Rigatto MH, Oliveira MS, Perdigão-Neto LV et al (2016) Multicenter prospective cohort study of renal failure in patients treated with colistin versus polymyxin B. Antimicrob Agents Chemother 60(4):2443–9
Zavascki AP, Nation R (2017) Nephrotoxicity of polymyxins: is there any difference between colistimethate and polymyxin B? Antimicrob Agents Chemother 61(3):e02319-16. https://doi.org/10.1128/AAC.02319-16
Freire MP, Garcia DO, Cury AP, Francisco GR, Santos NF, Spadão F et al (2019) The role of therapy with aminoglycoside in the outcomes of kidney transplant recipients infected with polymyxin and carbapenem-resistant Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 38(4):755–765
Satlin MJ, Kubin CJ, Blumenthal JS, Cohen AB, Furuya EY, Wilson SJ, Jenkins SG, Calfee DP (2011) Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 55(12):5893–5899
Funding
This study was funded by Fundo de Incentivo à Pesquisa (FIPE) do Hospital de Clínicas de Porto Alegre. APZ is a research fellow of the National Council for Scientific and Technological Development (CNPq), Ministry of Science and Technology, Brazil.
Author information
Authors and Affiliations
Contributions
Diógenes Rodrigues performed research, analyzed data, and wrote the paper; Giulia Soska Baldissera, Douglas Mathos, and Aline Sartori collected and analyzed data; Alexandre P. Zavascki analyzed data and wrote the paper; Maria Helena Rigatto conceived the study, performed research, analyzed data, and wrote the paper.
Corresponding author
Ethics declarations
Ethics approval
The project was carried out after its approval by the institution’s Research Ethics Committee number 2.687.149 and 2.476.428.
Consent to participate
As this was a retrospective study, the ethics committee waived the need for informed consent.
Consent for publication
All authors agree on the publication of this manuscript.
Competing interests
Alexandre P Zavascki receives research grants from Pfizer. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Mara Correa Lelles Nogueira
Rights and permissions
About this article
Cite this article
Rodrigues, D., Baldissera, G.S., Mathos, D. et al. Amikacin for the treatment of carbapenem-resistant Klebsiella pneumoniae infections: clinical efficacy and toxicity. Braz J Microbiol 52, 1913–1919 (2021). https://doi.org/10.1007/s42770-021-00551-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00551-x