Abstract
A review of studies by Safarian et al. and Kim show that the smelting reaction at equilibrium for ferromanganese and silicomanganese alloys is defined by the coupled reaction in the carbon-saturated condition \( 2\underline{\text{Mn}} + \, \left( {{\text{SiO}}_{ 2} } \right) \, = { 2 }\left( {\text{MnO}} \right) \, + \underline{\text{Si}} \). The behavior of slag at equilibrium is described by MnO and SiO2 as dependent variables and by non-reacting species, CaO, MgO, and Al2O3, as independent variables. Its characteristic behaviors are assessed in the pseudobinary system of MnO and SiO2 fixed by non-reacting components with analyses of ferromanganese and silicomanganese slag from one-month smelting operations. The behavior of fluid slag is defined by their melting temperature provided by phase equilibria of slag system. Liquidus of manganese slag systems by Kang et al., Zhao et al., and Roghani et al. is reconstructed in coordinates of MnO and SiO2 at fixed contents of CaO, MgO, and Al2O3. Conditions for fluid smelting slag are examined by referencing characteristic behaviors of smelting slag to liquidus of manganese slag systems to assess the effect of MgO and Al2O3. MgO facilitates fluid silicomanganese slag but would make ferromanganese slag viscous. Al2O3 makes silicomanganese slag fluid at Al2O3 content with 0.41 by weight ratio to SiO2. At higher contents of Al2O3, silicomanganese slag would be viscous with low MnO contents in slag. Al2O3 facilitates the development of fluid ferromanganese slag.
Similar content being viewed by others
Introduction
Manganese alloys are used for alloying and refining requirement of steels. Specifically, ferromanganese alloys are provided for the need of manganese element to promote strength of steels and prepared for having a high Mn content at about 80 wt pct (weight percent) Mn and a low Si content at about 0.5 wt pct Si. Silicomanganese alloys are applied for deoxidation of steels. Si element is a strong deoxidizer in steelmaking, and its potency is further enhanced when Si deoxidation is accompanied by Mn. For simultaneous deoxidation by Si and Mn, silicomanganese alloys are developed to have Si content at higher than 14 wt pct Si and Mn content at higher than 60 wt pct Mn.
At present, manganese alloys are carbothermically smelted in submerged arc furnaces by simultaneous reduction of MnO and SiO2, and the smelting process proceeds by designing slag to attain desired analyses of alloys. Production of ferromanganese alloys is carried out with low SiO2 and high MnO contents in slag, and that of silicomanganese alloys is with a high SiO2 content and the lowest practically possible MnO content in slag. As manganese is the valuable element, smelting operators recycle ferromanganese slag as a source of manganese for silicomanganese alloy and choose content of MnO in ferromanganese slag in a way to maximize the recovery of manganese through combined smelting processes of ferromanganese and silicomanganese alloys. If circumstances do not permit the recycle of ferromanganese slag through smelting silicomanganese alloy, the content of MnO in ferromanganese slag should be low enough to discard or throw away. Empirically, the content of MnO in discarding ferromanganese slag has been practiced with about 20 wt pct MnO or less, but such low MnO content in slag has caused severe furnace blows. Such problems in furnace operation have been attributed to the formation of silicate compounds with high melting temperatures. Smelting practitioners have alleviated such operational problems by modifying properties of slag with MgO and Al2O3. Design of smelting slag needs to address not only metallurgical requirements for smelting alloys from various raw materials but also fluidity of slag for safe and efficient operation of submerged arc furnaces.
Smelting operators have adopted the concept of slag basicity to design smelting slag. Constituents of slag are assigned either as basic or acid functioning components, and the slag basicity is defined by the ratio of sum of basic components to that of acid ones, [B = B/A = (Σ Basic oxides)/(Σ Acid oxides)], and used to characterize the behavior of smelting slag. Ferromanganese slag is designed to have a basicity of 2 by mole ratio basis with the intention of keeping all incoming SiO2 ingredient in slag and silicomanganese slag with a basicity of 1 to hold a part of incoming SiO2 ingredient in slag. Normally, Al2O3 is regarded as a neutral ingredient and not included in the assessment of slag basicity. Smelting experience has shown that slag basicity needs to be revised when changes are made in contents of MnO and gangue constituents such as MgO and Al2O3 in slag. Often, smelting operators have experienced an increased Si content in smelted ferromanganese alloy when the content of MnO in slag is lowered to the level less than 20 wt pct MnO or the content of Al2O3 is increased. Such incoherent experience appears to indicate that the current concept of basicity may not adequately describe the behavior of smelting slag involved in simultaneous reduction of MnO and SiO2. The concept of basicity takes constituent oxides including MnO and SiO2 as independent variables. Instead, MnO and SiO2 may be inter-dependent variables in smelting slag of ferromanganese and silicomanganese alloys.
This study is to examine the behavior of MnO and SiO2 in smelting slag by reviewing recent studies of smelting reactions for ferromanganese and silicomanganese alloys[1,2] and to characterize it with the use of data from submerged arc smelting operations. The fluidity of smelting slag is assessed by examining liquidus temperatures from recent studies of phase equilibria of ferromanganese and silicomanganese slag systems.[3,4,5,6,7,8]
Reaction Zones in Submerged Arc Furnaces
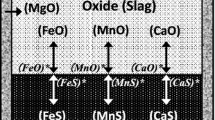
A typical submerged arc furnace is constructed to have a circular shell lined with refractory materials on top of a carbon lined hearth and to install three vertical carbon-based electrodes in a triangular configuration in the central location inside of the shell. Raw materials are charged to fill the space between electrodes and shell. The electrical energy is converted to heat at the tip of electrodes, and the region around electrodes attains smelting temperature. Hot CO gas from smelting reactions ascends through descending raw materials, and the ensuing heat exchange between them develops a temperature gradient through the burden mix. Temperature characterizes phases of raw materials and reactions taking place at various locations in furnaces. In general, four reaction zones are formed in submerged arc furnaces of smelting manganese alloys: (1) Pre-reduction zone is developed in the top region of burden mix. Moisture is driven off in this zone. Also, pre-reduction of various manganese oxides to MnO and Boudouard reaction take place in this zone in smelting ferromanganese alloys. In smelting silicomanganese alloys, the recycled ferromanganese slag is a major source of manganese, and the pre-reduction of manganese oxides and Boudouard reaction become minimum. (2) Fused oxide zone is developed in the next region at higher temperatures. As temperature is not high enough to smelt manganese and silicon, oxides in raw materials are fluxed and fused in this zone. (3) Coke bed zone is formed in the region of surrounding electrode tips. This region is rich in coke, which is mixed with fused oxides/slag. This mixture serves as an electric current carrier, and the resistance to current flow through it provides the necessary heat to smelt manganese and silicon. Smelting reactions take place in this zone. (4) Slag and alloy zone are developed in the region below the coke bed zone. Slag and alloy from smelting reactions are collected in the hearth, a lighter slag floating on top of a heavier alloy bath.
Smelting Reactions
Reactions in smelting processes of ferromanganese and silicomanganese alloys take place in a carbon-rich environment and involve carbothermic reductions of FeO, MnO, and SiO2. The prevailing reaction in carbothermic smelting process is the oxidation of carbon to form CO by Eq. [1].
where C(s) is carbon in coke, charcoal, or graphite, and O2(g) and CO(g) are oxygen and CO gas. It provides the prevailing oxygen potential in the smelting reaction system which dictates states in the reducing reactions of FeO, MnO, and SiO2.
Safarian et al.[1] showed in laboratory scale batch experiments that the reducing reactions proceed concurrently at different rates to develop alloy and slag, which are evolved by dynamics facilitated by difference in rates of reducing reactions. Smelting reactions for ferromanganese alloy are shown to proceed in two stages, where the initial main FeO reduction stage is followed by the later main MnO reduction stage. This study discusses the development of smelting reactions in each stage.
Main FeO Reduction Stage
During the main FeO reduction stage, FeO in slag is reduced by carbon in various reducing agents to produce Fe in liquid state and CO by Eq. [2].
where FeO in round bracket () indicates its state as solution in slag and C(s) is carbon in coke, charcoal, or graphite. The reducing reaction is observed to proceed at a rate three time higher than that for the reduction of MnO. As it is in carbon-rich environment, liquid iron becomes saturated with carbon, and the subsequent reaction proceeds to distribute Fe between carbon-saturated iron and slag as given by Eq. [3].
where underlines indicate ingredients in solution of carbon-saturated liquid iron. Safarian et al.[1] showed this reducing reaction is limited by the dissolution rate of C in iron, whereby it is high with coke and low with graphite and becomes in between with charcoal.
The reduction of MnO takes place concurrently by carbon to form liquid Mn by Eq. [4].
Safarian et al. showed that Mn in pure liquid state is unstable at smelting temperatures and evaporates preferentially when reduced with FeO free slag and pointed out that it needs to be stabilized by dissolution in liquid iron. In their experiment, the reduction of MnO is observed to proceed at about 1/3 of the rate of FeO reduction. As this takes place in the presence of carbon-saturated iron, liquid Mn dissolves readily in iron alloy as it is reduced, and the subsequent reaction proceeds to distribute Mn between carbon-saturated iron alloy and slag as given by Eq. [5].
The reduction of SiO2 does not take place during the main FeO reduction stage because the oxygen potential prevailing in the smelting reaction system is not sufficiently low, and the smelting reaction is defined by the distribution of Fe and Mn between carbon-saturated iron alloy and slag and described by Eq. [6], which is obtained by combining Eqs. [3] and [5].
This reaction assumes equilibrium between dissolved carbon C and CO(g), and proceeds until the FeO content in slag is depleted. The concentration of MnO in slag is observed to steadily increase during the main FeO reduction stage.
Main MnO Reduction Stage
Kim[2] showed that, right after depletion of FeO in slag, the prevailing oxygen potential is still not low enough for the reduction of SiO2, and the reduction of MnO continues to proceed as given by Eq. [5]. As the result of it, the concentration of Mn in alloy as well as that of SiO2 in slag increase steadily with an effect of lowering the prevailing oxygen potential in the smelting reaction system. Once it is low enough, the reduction of MnO and SiO2 proceeds concurrently at accelerated rates. The reduced Si is readily dissolved in carbon-saturated Mn-Fe alloy in presence. The subsequent reaction proceeds to distribute Si between alloy and slag as given by Eq. [7].
As the reduction of SiO2 takes place concurrently with that of MnO, therefore, the smelting reaction during the main MnO reduction stage is defined by the equilibrium distribution of Mn and Si between carbon-saturated Mn-Fe alloy and slag, and it is obtained by combining Eqs. [5] and [7].
Equation [8] is a coupled reaction involving alloy and slag, where the distribution of Si is balanced by that of Mn to maintain electroneutrality in the system of alloy and slag, while maintaining equilibrium between dissolved carbon, C, and CO(g). As it approaches to equilibrium, it characterizes smelting reaction for ferromanganese and silicomanganese alloys.
The smelting reaction by Eq. [8] shows that species of MnO and SiO2 in slag react with each other in the distribution of Mn and Si and that the SiO2 content in slag varies depending on the MnO content, i.e., they are inter-dependent variables. Because of ionic nature of slag, the distribution of Mn and Si incurs exchange of electron between reacting species. As Mn and Si are in different valance state in slag (Mn2+ and Si4+), the requirement of electroneutrality characterizes the relationship of SiO2 with MnO in smelting slag. On the other hand, species of CaO, MgO, and Al2O3 in slag do not come into distribution reaction and are variable independently. Therefore, the relationship of MnO and SiO2 in smelting slag would be appropriately assessed in the pseudobinary system fixed by non-reacting species.
Ferromanganese and Silicomanganese Slag from Smelting Operations
The behavior of MnO and SiO2 in the coupled reaction, Eq. [8], is discussed in relation to industrial practice by examining daily averaged analyses of slag from smelting operations of ferromanganese and silicomanganese alloys.
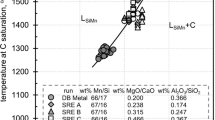
Plant data from DB Metal Company of one-month smelting operations are made available for the present discussion with four smelting practices: (1) SiMn (silicomanganese alloy) standard, (2) FeMn (ferromanganese alloy) standard, (3) FeMn discard slag, and (4) FeMn high alumina slag. These practices are made with contents of CaO, MgO, and Al2O3 at sufficiently high levels in slag, and it makes them relevant to the present discussion of their effect on the behavior of slag for safe and efficient smelting operations. Table I lists averaged analyses of alloys and slags in weight percent from smelting operations for a period of one month. The contents of MgO and Al2O3 are given as the weight ratios to wt pct CaO and to wt pct SiO2, respectively. This transformation for the contents of MgO and Al2O3 is to conform with the studies by Zhao et al.[4,5] and Roghani et al.[6,7,8] on phase equilibria in ferromanganese and silicomanganese slag systems.
Analyses of smelting slags are examined in coordinates of MnO and SiO2 contents. Their relationship as for ferromanganese or silicomanganese slag would be characterized by analyses of alloys in their respective classification and by contents of MgO and Al2O3 in slag. The FeO content in the slag is depleted.
Ferromanganese slag by three FeMn smelting practices is developed with ferromanganese alloys containing Mn and Si in the range of 77 to 81 wt pct Mn and about 0.5 wt pct Si and MgO and Al2O3 contents in the range of wt pct MgO/wt pct CaO = 0.17 to 0.216 and of wt pct Al2O3/wt pct SiO2 = 0.384 to 0.564, respectively. The content of SiO2 in ferromanganese slag is shown to increase with decreasing content of MnO in the range of SiO2 content at about 24 to 31 wt pct SiO2. This relationship appears to be defined mostly by analyses of FeMn alloys but to be rather insensitive to contents of MgO and Al2O3.
For silicomanganese slag, information is available only from SiMn standard practice for this study. It is processed with silicomanganese alloy containing 66 wt pct Mn and 16.7 wt pct Si and with MgO and Al2O3 contents by weight ratios of 0.200 and 0.366, respectively. The similar relationship of increasing SiO2 content with decreasing MnO content is observed to be developed in the range of SiO2 content at about 38 to 41 wt pct SiO2 for silicomanganese slag. The higher prevailing range of SiO2 content for the behavior of SiO2 with MnO in silicomanganese slag is derived from the distribution reaction of Si, whereby a higher Si content in alloy calls for a higher SiO2 content in slag. The observed relationship of increasing SiO2 content with decreasing MnO content in silicomanganese slag is defined primarily by analyses of SiMn alloy, but the effect of MgO and Al2O3 appears to be slight.
In the following discussion, the observed relationships of SiO2 with MnO are taken as characteristic behaviors of ferromanganese and silicomanganese smelting slag as given in the coupled Reaction [8] and referenced to phase equilibria of slag systems to examine conditions of fluid slag.
Phase Equilibria in Slag Systems
Profile of Fluid Slag by Phase Equilibria
Smelting operators have experienced that furnace operation becomes poor with severe furnace blows at times when smelting slag becomes viscous and tends to freeze quickly at tap. From such experience, an opinion among smelting practitioners has been developed that quick-freezing slag would form at melting temperature of about 1450 °C (1723 K), and fluid slag for smelting manganese alloys has been designed to have its melting temperatures to be under 1450 °C. The experienced quick-freezing slag problem is derived from freezing reaction of slag in a situation of two-phase mixture and attributable to a steep contour of liquidus in the field of manganosite and dicalcium silicate. Fluid slag would be realized by modifying liquidus surface to keep liquidus temperatures less than 1450 °C.
Phase diagrams of slag systems present the state of phase equilibria of liquid slag in conjugation of primary oxides and/or silicate compounds at liquidus temperatures. A profile of fluid slag may be defined as a liquid slag having liquidus temperature less than 1450 °C and applied to estimate melting temperatures of smelting ferromanganese and silicomanganese slag. As its scope will vary with constituents of slag systems, the concept of profiles of fluid slag would serve to assess the effect of non-reacting components on conditions for fluid slag.
Smelting slag for manganese alloys consists of MnO, CaO, and SiO2 as main ingredients, and necessary modifications of its properties are made by MgO and Al2O3. In this study, phase diagrams of MnO–CaO–SiO2,[3] MnO–CaO–MgO–SiO2–Al2O3,[4,5] and MnO–CaO–SiO2–Al2O3[6,7,8] systems are referenced to assess profiles of fluid slag for each slag system. Unfortunately, information from some of referenced slag systems[6,7] is uncertain at liquidus temperatures higher than 1400 °C. Instead of 1450 °C as the limiting melting temperature for fluid slag, information at 1400 °C is taken and adopted to define profiles of fluid slag. This choice of alternative limiting melting temperature is to maintain a consistency of treatment throughout this study, while it reasonably projects a potential viscous situation of liquid slag.
Phase equilibria of smelting slag need to be assessed in the context of coupled reaction, and liquidus surfaces of various slag systems[3,4,5,6,7,8] is reconstructed in the fields of primary phases relevant to ferromanganese and silicomanganese slag using MnO and SiO2 components as variables in this study.
Phase Equilibria in MnO–CaO–SiO2 System
Liquidus of MnO–CaO–SiO2 system by Kang et al.[3] is referenced for information of liquidus slag in conjugation with primary phases. The prevailing SiO2 content in smelting slag indicates that liquidus slag in the primary phase field of manganosite [(Mn,Ca)O] and of dicalcium silicate [α,α′-(Ca,Mn)2·SiO4] would account for the melting behavior of ferromanganese and silicomanganese slag, respectively. (Note: Chemical formula of silicate compounds and oxides are taken as reported in respective references.)
Liquidus temperature in the primary phase field of manganosite decreases from its melting point at 1842 °C as liquidus composition moves away from its stoichiometric composition, MnO. In the same manner, liquidus temperature in the primary phase field of dicalcium silicate decreases from its melting point at 2154 °C. Ultimately, liquidus composition reaches phase boundaries with neighboring primary phases, manganosite with tephroite [(Mn,Ca)2·SiO4] or dicalcium silicate and dicalcium silicate with tephroite or wollastonite [(Ca,Mn)·SiO3], respectively. The profile of fluid slag would be developed in the range of liquidus composition with liquidus temperatures less than 1400 °C in the primary phase fields of manganosite and dicalcium silicate.
Figure 1 reconstructs phase fields of manganosite and dicalcium silicate in coordinates of wt pct MnO and wt pct SiO2. Liquidus temperatures along these phase boundaries are presented by those at four invariant compositions, which assist to describe the behavior of liquidus temperature in the field of two primary phases. The isotherm at 1400 °C is listed as a broken line across the primary phase field of manganosite through dicalcium silicate, and it shows that the profile of fluid slag is developed in a narrow composition range along phase boundaries of manganosite and dicalcium silicate with tephroite. Also listed in Figure 1 are daily analyses of smelting slag from four smelting practices shown in Table I, and their melting temperatures are discussed by the profile of fluid slag.
Analyses of smelting silicomanganese slag by standard practice (SiMn standard) are shown to be described by compositions of liquidus slag along the isotherm of 1400 °C in the primary phase field of dicalcium silicate. Its proximity to the limiting melting temperature (1450 °C) indicates that silicomanganese slag by MnO–CaO–SiO2 system would be viscous at best. Analyses of smelting ferromanganese slag by three practices (FeMn standard, FeMn discard, and FeMn high Al2O3) are placed outside of the profile of fluid slag in the phase field of dicalcium silicate and manganosite. It indicates that ferromanganese slag by MnO–CaO–SiO2 system would have melting temperatures higher than 1400 °C and may be infusible. Such refractoriness of slag is attributed to high melting temperatures of dicalcium silicate and manganosite. A favorable profile of fluid slag may require to modify liquidus in the field of conjugating primary phases or to transform them. Addition of MgO and/or Al2O3 to the MnO–CaO–SiO2 system would facilitate their modification or transformation.
Phase Equilibria in MnO–CaO–MgO–SiO2–Al2O3 System
The effect of MgO on ferromanganese and silicomanganese slag is examined from the study of MnO–CaO–MgO–SiO2–Al2O3 system by Zhao et al.[4,5] They investigated phase equilibria in the quinary system by fixing MgO contents at two values with ratios of wt pct MgO/wt pct CaO = 0.17 and 0.25 and fixing Al2O3 content with the ratio of wt pct Al2O3/wt pct SiO2= 0.17. Two new primary phases, melilite [2CaO·(MgO,MnO,Al2O3)·2(SiO2,Al2O3)] and merwinite [3CaO·(Mg,Mn)O·2SiO2], are introduced by adding MgO and Al2O3 to the ternary system of MnO–CaO–SiO2.
Figure 2 reconstructs phase fields of melilite, merwinite, dicalcium silicate [α-2(Ca,Mg,Mn)O·SiO2], and manganosite [(Mn,Ca,Mg)O] and their phase boundaries in coordinates of wt pct MnO and wt pct SiO2. Two primary phases, melilite and merwinite, are developed in the quaternary system of CaO–MgO–SiO2–Al2O3 and evolved into the quinary system of MnO–CaO–MgO–SiO2–Al2O3 to form phase boundaries with wollastonite [(Ca,Mg,Mn)O·SiO2], tephroite [2(Mn,Ca,Mg)O,SiO2], dicalcium silicate, and manganosite. Behavior of phase boundaries is shown to be similar at two different levels of MgO content. Also listed are daily analyses of ferromanganese and silicomanganese slag of four smelting practices given in Table I.
Primary phase fields of melilite and merwinite are placed between that of wollastonite and dicalcium silicate in composition scales of MnO and SiO2. Compositions of smelting silicomanganese slag (SiMn standard) are presented within the primary phase field of melilite, indicating that melilite, instead of dicalcium silicate, would account for melting temperatures of smelting silicomanganese slag. This change is realized by melilite and merwinite which displace and reduce the phase field of dicalcium silicate. Tephroite is shown to form phase boundaries with them as well as with manganosite. Manganosite establishes phase boundaries with tephroite and dicalcium silicate as they would develop in the MnO–CaO–SiO2 system, but its phase boundary with tephroite extends further into the field of dicalcium silicate. Such modification offers a potential of lowering MnO content to about 18 to 29 wt pct MnO in ferromanganese slag.
Liquidus temperatures at invariant compositions are listed to describe the behavior of liquidus temperatures on liquidus surface of primary phases. The isotherm at 1400 °C with MgO content of wt pct MgO/wt pct CaO = 0.17 is shown to run through primary phase fields of manganosite, dicalcium silicate and/or merwinite. The isotherm with MgO content of wt pct MgO/wt pct CaO = 0.25 shows a similar behavior, but it is not listed for the sake of a concise presentation in this discussion. The profile of fluid slag is developed in the phase field of melilite and in a narrow composition range along phase boundaries with tephroite in the phase field of manganosite.
The profile of fluid slag developed in the prevailing range of SiO2 content for silicomanganese slag includes the entire phase field of melilite, and its liquidus temperature would be in the range of 1134 °C to 1372 °C. Analyses of smelting silicomanganese slag are placed within the phase field of melilite, indicating that their melting temperatures would be in the range of 1240 °C to 1290 °C. It is evident that such favorable melting temperatures for fluid slag are facilitated by the formation of melilite. On the other hand, analyses of smelting ferromanganese slag by three practices are placed just outside of the profile of fluid slag and run closely along the isotherm at 1400 °C through the phase field of manganosite toward the field of dicalcium silicate. It appears that the modification of liquidus in the field of manganosite and dicalcium silicate is slight. Ferromanganese slag by the quinary system would be viscous.
The quinary system of MnO–CaO–MgO–SiO2–Al2O3 by Zhao et al.[4,5] contains Al2O3 as well as MgO. A possible effect of Al2O3 needs to be ascertained in the quinary system. The study of phase equilibria in the MnO–Al2O3–SiO2 system by Roghani et al.[6] shows that tephroite (2MnO·SiO2) is stable at the content of Al2O3 less than 0.41 by the weight ratio to SiO2 and that galaxite (MnO·Al2O3) at the content higher than that. In the MnO–CaO–MgO–SiO2–Al2O3 system, tephroite phase is shown to be stable along with melilite and merwinite at the Al2O3 content with 0.17. If the same dynamic between Al2O3 and SiO2 prevails, the observed stability of tephroite with melilite in the quinary system is attributable to the MgO component because the content of Al2O3 is less than 0.41 in the referenced quinary system.[4,5]
Phase Equilibria in MnO–CaO–SiO2–Al2O3 System
The effect of Al2O3 on ferromanganese and silicomanganese slag is examined from the study of MnO–CaO–SiO2–Al2O3 system by Roghani et al.[7, 8] Phase equilibria in the quaternary system are investigated with Al2O3 contents at three levels, wt pct Al2O3/wt pct SiO2 = 0.41, 0.55, and 0.65. As they are prepared with high Al2O3 contents, tephroite is not reported in these systems. Instead of tehproite, manganosite ((Mn,Ca)O,) and α,α′-dicalcium silicate (2CaO·SiO2) are reported as stable primary phases along with anorthite (CaO·Al2O3·2SiO2), gehlenite (2CaO·Al2O3·SiO2), and galaxite (MnO·Al2O3) in the quaternary system.
Figure 3 reconstructs phase fields of anorthite, gehlenite, galaxite, dicalcium silicate, and manganosite and their phase boundaries in coordinates of wt pct MnO and wt pct SiO2. Anorthite develops in the region of high SiO2 content to have phase boundaries with neighboring gehlenite and galaxite. Gehlenite is placed between anorthite and dicalcium silicate in the coordinate of SiO2 content and develops phase boundaries with anorthite, galaxite, dicalcium silicate, and manganosite. Dicalcium silicate is placed in a region of low SiO2 content and forms phase boundaries with gehlenite and manganosite, and its field is noticeably reduced in coordinates of SiO2 and MnO contents. Manganosite is shown to develop in low levels of SiO2 content also by extending the phase boundary with gehlenite into the field of dicalcium silicate. Such extension of the phase boundary of manganosite provides a possibility of lowering MnO content to about 19 to 23 wt pct MnO in ferromanganese slag. Also shown are analyses of ferromanganese and silicomanganese slag by the four smelting practices listed in Table I. Compositions of smelting silicomanganese slag are placed in the phase field of anorthite, and analyses of smelting ferromanganese slag are distributed in phase fields of manganosite and gehlenite around their shared phase boundary.
Liquidus temperatures at invariant compositions are listed also in Figure 3 to describe the behavior of liquidus temperatures on liquidus surface of primary phases. The isotherms at 1400°C are presented at two contents of Al2O3 with ratios of wt pct Al2O3/wt pct SiO2 = 0.41 and 0.65. (Note: Isotherms are denoted as temperatures followed by Al2O3 content in parenthesis.) They run through the phase fields of anorthite as well as manganosite and gehlenite. Two profiles of fluid slag are at work, one in the field of anorthite for silicomanganese slag and the other in fields of manganosite and gehlenite for ferromanganese slag.
For a closer examination, Figure 4 reproduces the liquidus of anorthite with additional isotherms at 1250 °C, 1300 °C, and 1350 °C at three levels of Al2O3 content. Isotherms are shown to develop as arch-shaped curves in the field of anorthite, and the trajectory of liquidus compositions for isotherm apexes defines the field of anorthite into hypo- and hyper-anorthite. Analyses of smelting silicomanganese slag are placed in the field of hypo-anorthite. Liquidus temperatures in the field of hypo-anorthite are shown to decrease with increasing MnO content and with decreasing SiO2 content. The profile of fluid slag is defined by liquidus along the isotherm at 1400 °C, isotherm apexes, and the phase boundary of hypo-anorthite with gehlenite. Such defined profile of fluid slag is shown to shift downward in the coordinate of SiO2 content as the content of Al2O3 content increases, and, consequently, an increase of Al2O3 content would raise melting temperatures of silicomanganese slag at a given composition in the quaternary system.
At the content of Al2O3 with 0.41 (by weight ratio to SiO2), analyses of smelting silicomanganese slag are shown to follow closely the phase boundary of anorthite with gehlenite. This indicates that melting temperatures of smelting silicomanganese slag would be in the range of 1240 °C to 1300 °C and that smelting silicomanganese slag would be fluid. At increased content of Al2O3 with 0.55, analyses of smelting silicomanganese slag are placed in the region of high liquidus temperatures in the field of hypo-anorthite, and their melting temperatures are estimated to be in the raised range of 1310 °C to 1365 °C. The increasing relationship of SiO2 content with decreasing MnO in silicomanganese slag indicates that melting temperature of silicomanganese slag would increase further to 1400 °C at about 3 wt pct MnO. At higher content of Al2O3 with 0.65, melting temperature of silicomanganese slag are estimated to be in the range of 1350 °C to 1395 °C. A similar trend suggests that the melting temperature of smelting slag would be 1400 °C at about 9 wt pct MnO. It implies that a raised Al2O3 content to levels of 0.55 to 0.65 may make silicomanganese slag viscous with low MnO contents.
Again, for a detailed examination in the prevailing range of ferromanganese slag, Figure 5 reproduces liquidus of manganosite and gehlenite with the additional isotherms at 1250 °C, 1300 °C, and 1350 °C at contents of Al2O3 with 0.41, 0.55, and 0.65. Phase equilibria of manganosite with gehlenite is characterized by two invariant points, one developed with galaxite and the other with dicalcium silicate. Invariant points with galaxite are developed at 36.3 wt pct MnO and 1198 °C, 42.3 wt pct MnO and 1155 °C, and 43.2 wt pct MnO and 1223 °C as the content of Al2O3 increases from 0.41, to 0.55 through 0.65, respectively. Compositions of ferromanganese slag by standard practice evolve closely to these invariant points. Invariant points with dicalcium silicate are developed at 23.2 wt pct MnO and 1400 °C, 20.4 wt pct MnO and 1365 °C, and 19.2 wt pct MnO and 1485 °C (estimated by extrapolation) as the content of Al2O3 increases from 0.41 to 0.55 through 0.65, respectively. Compositions of ferromanganese slag by discard slag practice are developed with similar invariant MnO contents but with raised SiO2 contents along isotherm at 1400 °C in the field of gehlenite. Analyses of ferromanganese slag by high Al2O3 slag practice are presented along isotherms of 1300 °C to 1350 °C in the field of gehlenite. In general, Al2O3 facilitates favorable liquidus in the field of manganosite and gehlenite for fluid ferromanganese slag.
Effect of Al2O3 on Si Content in Ferromanganese Alloys
Distribution of Si between ferromanganese alloys and slag is driven by the chemical potential of SiO2 in slag, and its reaction may be defined by the ratio of wt pct Si to wt pct SiO2.
Distribution ratios of Si for smelting process of ferromanganese alloys are developed from daily analyses of ferromanganese alloys and slags by three smelting practices listed in Table I. Figure 6 lists them to assess the effect of Al2O3 in ferromanganese slag. It shows that the distribution of Si is independent of Al2O3 content when ferromanganese slag is developed in the field of manganosite but increases with increasing Al2O3 content in the field of gehlenite. It indicates that Al2O3 would promote the distribution of Si but does so at the SiO2 content higher than threshold levels of SiO2. The threshold would be developed in liquid slag along the phase boundary of manganosite with gehlenite.
The chemical potential of SiO2 in smelting slag would increase with increase of the content of SiO2 and by its interaction with Al2O3 as well. Both SiO2 and Al2O3 are in terminal composition region of smelting slag in the phase field of manganosite. As they appear to behave as dilute solutes which do not interact in liquid MnO solvent, the content of Si is shown to depend only on that of SiO2 in smelting slag. However, once their contents are increased to be in the primary phase field of gehlenite, Al2O3 appears to preferentially associate with CaO and to enhance the chemical potential of SiO2. This hypothesis needs to be verified by a further study.
Conclusion
By identifying MnO and SiO2 as inter-dependent variables and CaO, MgO, and Al2O3 as independent variables, ferromanganese and silicomanganese slag from smelting operation are referenced to phase equilibria of MgO and Al2O3 containing MnO–CaO silicate systems[3,4,5,6,7,8] to examine conditions for fluid smelting slag.
MgO makes melilite stable, and silicomanganese slag develops in the field of it. Its favorable profile of fluid slag provides melting temperature of silicomanganese slag to be in the range of 1240 °C to 1290 °C. Ferromanganese slag develops in the field of manganosite. However, MgO modifies the liquidus surface of manganosite only in a limited extent. Resulting melting temperatures of ferromanganese slag would be higher than 1400 °C, and slag would be viscous.
Al2O3 provides for silicomanganese slag to be developed in the field of anorthite, specifically in the field of hypo-anorthite. Liquidus temperatures in the field of hypo-anorthite are low at the Al2O3 content with 0.41 by weight ratio to SiO2 and makes silicomanganese slag fluid. At higher contents of Al2O3, liquidus temperatures are high, and silicomanganese slag would be viscous with low MnO contents. Al2O3 facilitates also stability of gehlenite which modifies liquidus surface of manganosite considerably, and ferromanganese slag is developed in the field of gehlenite or manganosite. When the content of MnO in slag is higher than about 35 wt pct MnO and that of Al2O3 is 0.41, the phase field of manganosite provides a favorable profile of fluid slag. Otherwise, the field of gehlenite enables the development of fluid smelting ferromanganese slag.
References
1. J. Safarian, L. Kolbeinsen, M. Tangstad, and G. Tranell: Metall. Mater. Trans. B, 2009, vol.40B, pp.929-939
P.P. Kim, PhD thesis, DMSE, Norwegian University of Science and Technology (NTNU), Trondheim, Norway (2018)
3. Y. Kang, I. Jung, S.A. Decterov, A.D. Pelton and H. Lee: ISIJ International, 2004, vol.44, pp.965-974
4. B. Zhao, E. Jak and P.C. Hayes: ISIJ International, 2005, vol.45, pp.1019-1026
5. B. Zhao, E. Jak and P.C. Hayes: ISIJ International, 2006, vol.46, pp.1594-1602
6. G. Roghani, E. Jak, and P. Hayes: Merall. Mater. Trans. B, 2002, vol.33B, pp.827-838
7. G. Roghani, E. Jak, and P. Hayes: Metall. Mater. Trans. B, 2002, vol.33B, pp.839-849
8. G. Roghani, E. Jak, and P. Hayes: Metall. Mater. Trans. B, 2003, vol.34B, pp.173-182
Acknowledgments
The authors thank DB Metal Company for providing data from smelting operation. One of the authors, Leiv Kolbeinsen, gratefully acknowledges the financial support from the Research Council of Norway and the partners of the SFI Metal Production (Centre for Research-based Innovation, 237738).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted February 1, 2021; accepted May 28, 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, Y.E., Kolbeinsen, L. Behavior of Slag in Ferromanganese and Silicomanganese Smelting Process. Metall Mater Trans B 52, 3142–3150 (2021). https://doi.org/10.1007/s11663-021-02242-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-021-02242-2