Access to Resources Shapes Sex Differences Between Caring Parents
- 1Département des Sciences Biologiques, Université du Québec à Montréal, Montréal, QC, Canada
- 2Institute of Evolutionary Biology, School of Biological Sciences, University of Edinburgh, Edinburgh, United Kingdom
In species where both parents cooperate to care for their joint offspring, one sex often provides more care than the other. The magnitude of such sex differences often varies both between and within species and may depend on environmental conditions, such as access to resources, predation risk and interspecific competition. Here we investigated the impact of one such environmental variable – access to resources for breeding – on the magnitude of sex differences in parental care in the burying beetle Nicrophorus vespilloides. This species breeds on the carcasses of small vertebrates, which are the sole food source for parents and offspring during breeding. We manipulated access to resources by providing pairs with mouse carcasses from a broad mass range (3.65–26.15 g). We then monitored subsequent effects on the duration and amount of care provided by males and females, male and female food consumption and weight change during breeding, and larval traits related to offspring performance. We found that males increased their duration of care as carcass mass increased, while females remained with the brood until it had completed its development irrespective of carcass mass. There were thus more pronounced sex differences in parental care when parents had access to fewer resources for breeding. Overall, our findings show that sex differences between caring parents vary depending on access to resources during breeding. The finding that males extended their duration of care on larger carcasses suggests that access to more resources leads to a shift toward more cooperation between caring parents.
Introduction
Biparental care occurs when male and female parents cooperate to care for their joint offspring. It is the predominant pattern of care in birds (Cockburn, 2006) but has also evolved in a small number of mammals, amphibians, fishes, and arthropods (Balshine, 2012; Trumbo, 2012). Biparental care is often associated with sex differences in the amount or duration of care with females usually making greater contributions than males (Kokko and Jennions, 2012; West and Capellini, 2016). For example, females provide more care than males in red-winged blackbirds (Whittingham, 1989), house sparrows (Schwagmeyer et al., 2008), oldfield mice (Margulis, 1998), convict cichlids (Lavery and Keenleyside, 1990), and burying beetles of the genus Nicrophorus (Smiseth and Moore, 2004; Trumbo, 2007). Such sex differences in care reflect differences between males and females in the benefits and/or costs of care. For example, in the cichlid fish Herotilapia multispinosa, where males desert the brood earlier than females, males presumably incur higher costs of care because they can mate with a new partner quicker, and thus lose more mating opportunities than females when continuing to provide care (Keenleyside, 1983). The magnitude of such sex differences varies both between and within species, and this variation would depend on environmental conditions that have a differential impact on the costs and/or benefits of care to males and females. For example, prior work shows that the magnitude of sex differences in parental care varies with ambient temperatures (e.g., Vincze et al., 2013) or the intensity of interspecific competition (e.g., Hopwood et al., 2015). Variation in access to resources is likely to be a key environmental condition in this respect given that such variation may have a differential impact on the benefits and/or costs of care to males and females (e.g., Eldegard and Sonerud, 2010). In order to advance our understanding of variation in the magnitude of sex differences in parental care, there is now a need for experiments that manipulate access to resources and then monitor effects on male and female care.
Access to resources may also impact on sexual conflict between parents over parental care (Lessells, 2012). Sexual conflict arises because the benefits in terms of enhanced offspring fitness result from the combined effort of the two parents, whilst the costs in terms of reduced future survival and reproduction depend on each parent’s personal effort (Trivers, 1972; Chase, 1980). As such, biparental care involves a balance between cooperation and conflict, and any shift in this balance could be detected as a change in the frequency and/or duration of biparental care (Westneat and Sargent, 1996; Lessells and McNamara, 2012; Johnstone and Savage, 2019). Greater access to resources may reduce the benefits of biparental cooperation in species where parents provision food to the offspring. When food is abundant, females can provision more food to the brood on their own, thereby reducing the benefits to males from assisting females (Crook, 1963; Leisler et al., 2002; Barve and La Sorte, 2016). Yet, on the other hand, greater access to food may increase the benefits of biparental cooperation in species where parents protect the offspring from predators or conspecific intruders. For example, if greater access to food increases the risk of nest predation or infanticide by conspecific intruders (e.g., Wilson and Fudge, 1984; Robertson, 1993), there may be an increase in the benefits to the male from assisting the female when food is more abundant. Thus, experiments that manipulate access to resources should also monitor effects on the frequency and/or duration of biparental care relative to uniparental care.
We used the burying beetle Nicrophorus vespilloides to investigate how availability of resources alters the magnitude of sex differences in care and shifts the balance between cooperation and conflict. Burying beetles of the genus Nicrophorus are ideal to address these issues because they breed on carcasses of small vertebrates that vary considerably in mass (Müller et al., 1990; Smiseth and Moore, 2002). The vertebrate carcass used for breeding is the sole source of food for both developing larvae and caring parents (Scott and Traniello, 1990; Scott, 1998; Pilakouta et al., 2016). Thus, it is straightforward to manipulate the availability of resources by simply providing parents with carcasses of variable masses (Smiseth et al., 2014). Unlike birds where two parents can supply more food to the brood than a single parent, the supply of food in burying beetles is limited by the size of the carcass and should not be dependent on the number of parents attending the brood. These species show facultative biparental care, whereby male and female parents cooperate to varying degrees by caring for the developing larvae (Eggert et al., 1998; Scott, 1998). Thus, a shift in the balance between cooperation and conflict could be detected as a change in the duration of biparental care. Both female and male parents provide care by provisioning pre-digested carrion to the larvae and defending the carcass and the brood from conspecific intruders (Eggert et al., 1998; Scott, 1998). Females spend more time on parental care (e.g., Smiseth et al., 2005; Georgiou-Shippi et al., 2018) and care for longer than males (Bartlett, 1988; Ford and Smiseth, 2016), yet it is unclear what impact variation in carcass mass would have on the magnitude of such sex differences in care. Prior work also shows that there are synergistic effects of biparental cooperation, and that that these often outweigh the detrimental effects of sexual conflict (Pilakouta et al., 2018). However, it is unclear how variation in carcass mass would impact on the balance between cooperation and conflict.
Our aim was to test for effects of variation in carcass mass on sex differences in care and the balance between cooperation and conflict. We provided breeding pairs with mouse carcasses of variable mass (3.65–26.15 g). We then monitored subsequent effects on the duration of biparental care, sex differences in the duration of male and female care and the amount of time spent providing care by males and females, resource consumption and weight change by males and females during breeding, and brood size and mean larval mass at the time of larval dispersal. We predicted that sex differences in parental care would be more pronounced as carcass mass decreased. The reason is that the benefits of male care should be lower as carcass size decreases given that smaller carcasses are less valuable to conspecific intruders. We also predicted that females would respond less to an increase in carcass mass than males in terms of carrion consumption and weight gain given that caring parents have greater access to the carcass as a food source for themselves (Pilakouta et al., 2016). This is because females are predicted to remain at the carcass for a similar amount of time regardless of carcass size, whereas males are predicted to provide care for longer on larger carcasses, thereby giving them more opportunities to consume from the carcass (Keppner et al., 2018). As argued above, an increase in carcass mass may lead to a shift toward either more conflict or more cooperation between parents. The latter prediction seems more likely in N. vespilloides given that larger carcasses are more valuable to conspecific intruders, and that two parents are more efficient at protecting their brood against intruders than single ones (Trumbo, 1991). In the wild, breeding success relies greatly on the attendance of both parents (e.g., Scott and Traniello, 1990; Trumbo, 1991, 2006, 2007; Eggert and Sakaluk, 2000; Hopwood et al., 2015), and we therefore used the duration of biparental care as a proxy for the level of cooperation between the male and female parents.
Materials and Methods
Origin and Rearing of Experimental Beetles
We used virgin beetles from an outbred laboratory population maintained for at least four generations at the University of Edinburgh. The laboratory population descended from beetles that originally were collected in Hermitage of Braid and Blackford Hill Local Nature Reserve, Edinburgh, United Kingdom. We maintained non-breeding adult beetles in individual transparent plastic containers (12 cm × 8 cm × 2 cm) filled with moist soil, under a constant temperature (20°C) and a 16:8 h light:dark photoperiod. We fed non-breeding adult beetles a small piece of organic beef twice a week.
Experimental Design and Procedures
We designed a laboratory experiment where we tested for effects of variation in carcass mass on the magnitude of sex differences in care and the balance between cooperation and conflict by manipulating the mass of the carcass that pairs were provided with at the start of breeding. We started the experiment by pairing virgin females with a randomly assigned, unrelated, virgin male partner. To ensure that all beetles were sexually mature and to avoid any confounding effect of age on parental traits, we used males and females aged between 10 and 28 days following eclosion. We weighed all males and females at this stage to record their pre-breeding mass. To initiate breeding, each pair was moved to a larger, transparent container (17 cm × 12 cm × 6 cm) filled with 1 cm of moist soil and provided with a previously frozen mouse carcass (Livefoods Direct, Sheffield). We randomly assigned each pair with a mouse carcass that weighed between 3.65 and 26.15 g (mean ± SE = 13.41 ± 0.396 g). This mass range matches that used by our study species under natural (2–30 g; Müller et al., 1990) and laboratory conditions (2–40 g; Smiseth and Moore, 2002). Varying the size of the carcass is a well-established protocol in burying beetle species allowing us to manipulate access to the breeding resource (e.g., Bartlett, 1988; Eggert and Müller, 1992; Trumbo, 1992; Xu and Suzuki, 2001; Smiseth and Moore, 2002; Creighton et al., 2009; De Gasperin and Kilner, 2015; Magneville et al., 2018).
From the day of mating onwards, we checked each container daily for the presence of eggs. We did this to record the day on which the first eggs were laid. Females lay their eggs in the soil surrounding the carcass, and most eggs are visible from the bottom of the transparent container in a thin layer of soil (Monteith et al., 2012), as used in our experiment. We counted the eggs 2 days after the onset of egg-laying (i.e., the day preceding the time of hatching of the first eggs in the clutch) and used the number of eggs as a measure of clutch size. On the following day, when the eggs started to hatch, we counted the number of newly hatched larvae, using this as a measure of brood size on the day of hatching. Given that females lay their eggs asynchronously over a mean period of 27 h (Müller, 1987; Smiseth et al., 2006), the final brood size may be larger than brood size on the day of hatching.
We recorded shifts in the balance between cooperation and conflict by monitoring the duration of biparental care. We checked the containers daily from the time of mating until the time of dispersal, recording whether the male and the female were still present on the carcass or whether either of them had deserted the brood. We scored the male or the female as having deserted the brood if the male or the female was absent from the crypt (i.e., the depression in the soil surrounding the carcass) on two consecutive days. We removed any parent that had deserted the brood from the breeding container to prevent the deserting parent from posing a risk to the brood. Note that we refrained from removing any deserting parent before we conducted the behavioural observations 24 h after hatching (see details below). Removing a deserting parent matches what would happen under natural conditions given that deserting parents leave the carcass permanently (Scott and Traniello, 1990). We removed deserting parents because it may kill larvae when maintained with the brood beyond the time of desertion (Authors’ personal observation). We weighed any deserting parent to record information on weight change during breeding (see below). We recorded the duration of biparental care as the number of days from mating until one of the parents deserted the brood. If both parents cared for the brood until the larvae dispersed from the carcass, we recorded the duration of biparental care as the number of days from mating until the larvae dispersed from the carcass (normally 7 days; Scott, 1998; Grew et al., 2019).
We monitored the behaviour of parents on the day after the first eggs had hatched to estimate the amount of time that each parent spent providing care and consuming resources. This time point corresponds to the peak of parental food provisioning to larvae in this species (Smiseth et al., 2003). We conducted behavioural observations for 30 min under red light, recording the behaviour of both parents at 60 s intervals in line with established protocols (e.g., Smiseth and Moore, 2002, 2004; Pilakouta et al., 2018). Note that, apart from the light, laboratory conditions were identical during behavioural observations (i.e., constant 20°C temperature). We recorded whether each parent was provisioning food, defined as any mouth-to-mouth contact between a parent and at least one larva, maintaining the carcass, defined as excavation of the soil around the carcass or coating the carcass with exudates, or in near proximity to the brood, defined as whenever a parent was at a distance from larvae that was approximately equal to or shorter than its pronotum length (e.g., Smiseth and Moore, 2002, 2004). We recorded time spent consuming carrion as any instances where a parent was feeding within the crater (i.e., the opening on the top of the carcass; e.g., Pilakouta et al., 2016). Feeding from the crater generally reflects that parents consume carrion for their own use or to regurgitate to the larvae (Pilakouta et al., 2016), although it can sometimes reflect that parents are enlarging the crater (e.g., Shukla et al., 2018). At each scan, we also recorded the number of larvae that were begging to a parent. We then calculated the average proportion of time spent begging per larva in the brood as B = (∑b/n)/p, where ∑b is the cumulative number of begging events during the 30-min observation period, n is the brood size at the time of observation, and p is the number of scans during which a parent was in close proximity to the brood.
We left experimental broods undisturbed until the larvae dispersed from the carcass. At the time of dispersal, we counted the number of larvae to gain information on brood size and we weighed the whole brood to calculate mean larval mass as total brood mass divided by brood size. We also weighed each parent again at dispersal and calculated relative weight change during breeding as the difference in body mass measured at dispersal (or removal) and pre-breeding mass, divided by pre-breeding mass. In this species, parents feed from the carcass during breeding (Pilakouta et al., 2016), and parental weight change is used as a proxy for investment in future reproduction (Creighton et al., 2009; Billman et al., 2014; Gray et al., 2018).
Statistical Analysis
All statistical analyses were conducted using R version 3.6.0 (R Core Team, 2019) loaded with the packages car (Fox et al., 2017), MASS (Ripley et al., 2017), and glmmTMB (Brooks et al., 2017). We analysed data on the shift between cooperation and conflict between the two parents as a number of days of biparental care using a generalised linear model (GLM) assuming a Poisson error structure and including carcass mass as the only fixed effect. We analysed data on sex differences in the duration of care using GLMs assuming Poisson error structures. We verified the absence of over-dispersion and the good fit of the models by plotting the residuals using the “simulateResiduals” function of the DHARMa package in R (Hartig, 2017). To analyse data on sex differences in parental behaviour on the day after hatching (i.e., the amount of time spent provisioning food to the brood, maintaining the carcass, and consuming carrion), we used GLMs with zero-adjusted binomial distributions to account for zero-inflation and over-dispersion. We used linear models to analyse data on parental weight change over breeding. In all other models, we included carcass mass, the sex of the focal parent and, to test for potential sex-specific responses to resource availability, the interaction between carcass mass and sex. We also tested whether potential effects of carcass mass on parental behaviours on the day of hatching were fully or partially driven by clutch size or brood sizeat the time of observation or brood size. The reason for this is that parents adjust the amount of care that they provide to the number of offspring in the brood (Smiseth et al., 2007; Ratz and Smiseth, 2018), and that brood size covaries with carcass size (Bartlett and Ashworth, 1988; Smiseth et al., 2014). To determine whether any overall effect of carcass mass was causally linked to variation in clutch size or brood size, we first ran each model excluding clutch size or brood size at the time of observation and then compared this model to a full model that included clutch size or brood size at the time of observation as a fixed effect. We used the “Anova” function of the R package car (Fox et al., 2017) to obtain χ2 and p-values provided in tables and the “summary” function to obtain the estimates, z-values and p-values provided in the text.
For our analyses on offspring behaviour and performance, we used a GLM assuming a binomial error structure to analyse data on the average time spent begging by individual larvae, a GLM assuming a negative binomial error structure to analyse data on brood size at dispersal, and a linear model to analyse data on mean larval mass at dispersal. All models included carcass mass as a fixed effect. We also examined the effect of biparental cooperation on offspring performance by including the duration of biparental care as a covariate in models on brood size and mean larval mass at dispersal. As described above, we first excluded clutch size or brood size at the time of observation from the models and then ran each model again including clutch size or brood size at the time of observation as an additional fixed effect. As described above, χ2 and p-values were obtained using the “Anova” function and estimates, z-values and p-values were obtained using the “summary” function in R.
Results
Sex Differences in Duration of Care
There was a significant effect of the interaction between the sex of the focal parent and carcass mass on the duration of care (Table 1). This interaction effect reflected that males provided care for longer as carcass mass increased, whilst females tended to provide care until the time of larval dispersal regardless of carcass mass (Figure 1A; sex × carcass mass: estimate = 0.016, SE = 0.006, z = 2.59, P = 0.010). Thus, as predicted, sex differences in parental care became more pronounced as carcass mass decreased. There was no significant main effect of carcass mass on the duration of female care (Table 1). However, males deserted the brood earlier, and thus provided care for a shorter period of time, than females as carcass mass decreased [Table 1; mean ± SE duration of care from the day of mating: male = 4 ± 0.15 days, female = 7 ± 0.13 days; estimate (male versus female) = −0.64, SE = 0.103, z = −6.21, P < 0.001].
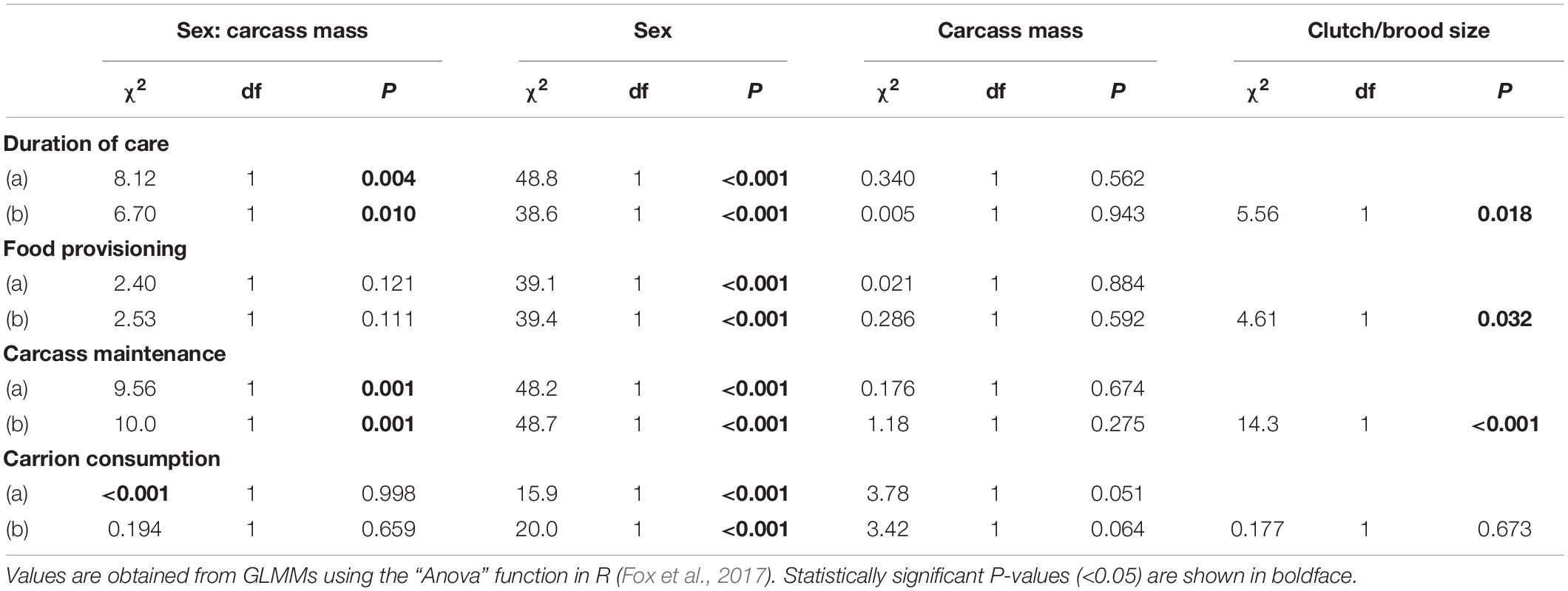
Table 1. Effects of the interaction between sex of the focal parent and carcass mass on the duration of uniparental care when clutch size excluded (a) and included (b). Effects of the interaction between sex of the focal parent and carcass mass on time spent provisioning food to the brood, maintaining the carcass and consuming carrion when brood size at the time of observation was excluded (a) and included (b).
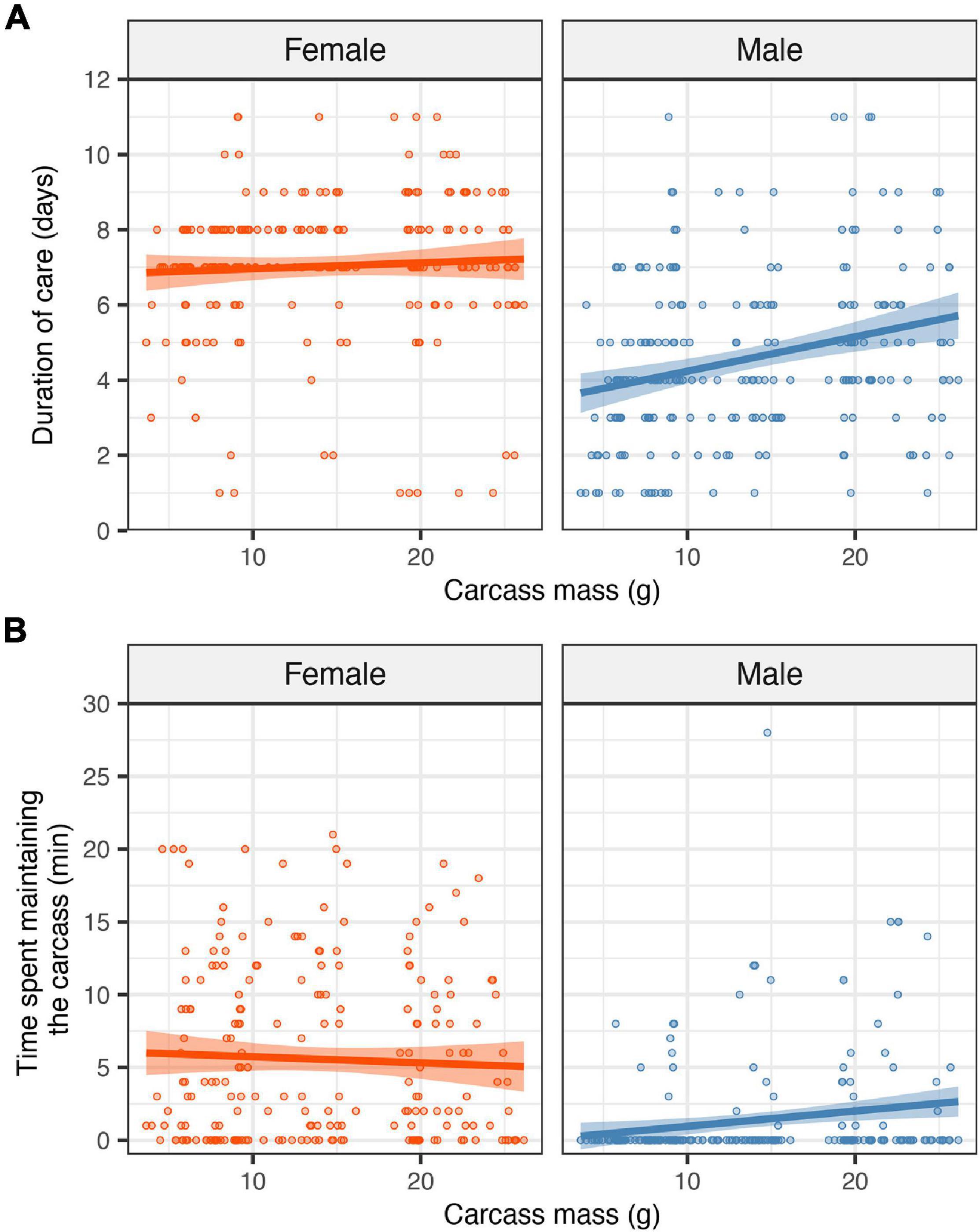
Figure 1. Effects of carcass mass on the duration of female and male parental care (A) and on the time each parent spent on carcass maintenance (B). Filled circles represent individual data points, lines represent linear regression lines and shaded ribbons the 95% confidence intervals.
Sex Differences in Amount of Care
There was no effect of the interaction between the sex of the focal parent and carcass mass on the amount of time parents spent provisioning food to the brood on the day after hatching (Table 1). There was no significant main effect of carcass mass on the amount of time spent provisioning food to the brood (Table 1). Males spent significantly less time, on average, provisioning food to the larvae than females [mean ± SE time spent provisioning food out of 30 min: male = 0.74 ± 0.18 min, Female = 4.4 ± 0.3 min; estimate (male versus female) = −4.59, SE = 0.732, z = −6.27, P < 0.001].
The interaction between the sex of the focal parent and carcass mass had a significant effect on the time spent maintaining the carcass (Table 1 and Figure 1B), reflecting that males spent more time maintaining the carcass as carcass mass increased whereas carcass mass had no noticeable effect on the amount of time spent maintaining the carcass by females (sex × carcass mass: estimate = 0.148, SE = 0.046, z = 3.17, P = 0.001). There was no main effect of carcass mass on time spent maintaining the carcass (estimate = −0.031, SE = 0.028, z = −1.09, P = 0.275). However, females spent significantly more time maintaining the carcass than males [mean ± SE time spent on carcass maintenance out of 30 min: male = 1.4 ± 0.23 min, female = 5.6 ± 0.38 min; estimate (male versus female) = −5.34, SE = 0.764, z = −6.98, P < 0.001].
Given that the number of offspring in the brood is positively correlated with carcass mass (r = 0.20, t = 3.0365, df = 204, P = 0.002), we compared models where we excluded and included clutch size or brood size at the time of observation as fixed effects to analyse the duration and the amount of parental care, respectively. We did this to disentangle the causal effects of carcass mass and the number of offspring in the brood on parental behaviour. Excluding or including clutch size or brood size at the time of observation did not change the effect of carcass mass (Table 1), suggesting that the effects of carcass mass on the behaviour of the parents were independent of any potential effects due to the number of offspring in the brood.
Sex Differences in Carrion Consumption and Weight Change
There were no significant effects of the interaction between the sex of the focal parent and carcass mass and no significant main effects of carcass mass on the amount of time spent consuming carrion by the female or male parent measured on the day after hatching (Table 1). However, females spent significantly more time consuming carrion than males [mean ± SE time spent consuming out of 30 min: male = 0.87 ± 0.21 min, female = 3.6 ± 0.33 min; estimate (male versus female) = −3.69, SE = 0.825, z = −4.47, P < 0.001].
There was a significant effect of the interaction between the sex of the focal parent and carcass mass on weight change over the breeding attempt (F1,368 = 0.046, P = 0.027), reflecting that carcass mass had a stronger positive effect on female weight change than on male weight change (Figure 2; mean ± SE weight change: male = 0.027 ± 0.006 g, female = 0.068 ± 0.007 g). Parents gained more mass as carcass mass increased (estimate = 0.005, SE = 0.001, t = 4.52, P < 0.001). There was no significant difference between male and female parents in the average weight change (F1,368 = 0.0009, P = 0.754). Excluding or including clutch size at the time of observation did not change the effect of carcass mass, suggesting that any effect of carcass mass on the weight gain of parents was independent of any potential effects due to the number of offspring in the brood.
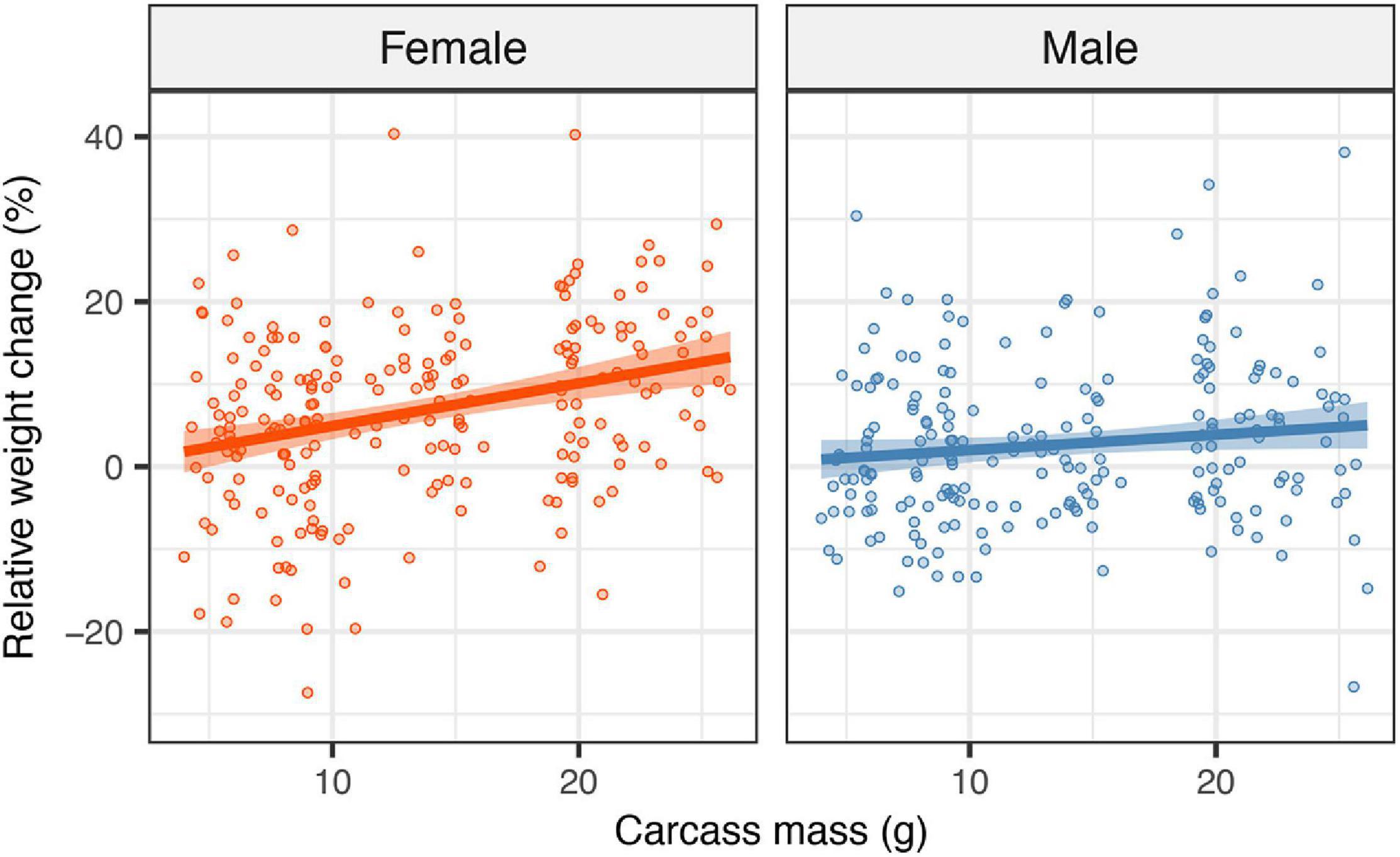
Figure 2. Effects of carcass mass on the weight change of each parent. Filled circles represent individual data points; lines represent linear regression lines and shaded ribbons the 95% confidence intervals.
Balance Between Cooperation and Conflict
The duration of biparental care increased by approximately 0.6 days for each additional 10 g of carcass (Figure 3; estimate = 0.012, SE = 0.005, z = 2.28, P = 0.022), supporting the prediction that an increase in carcass mass was associated with a shift toward more cooperation between parents. Clutch size had a significant positive effect on the duration of biparental care (estimate = 0.007, SE = 0.003, z = 2.09, P = 0.037). Including clutch size in the model, however, did not change the direction or the significance of the effect of carcass mass on the duration of biparental care.
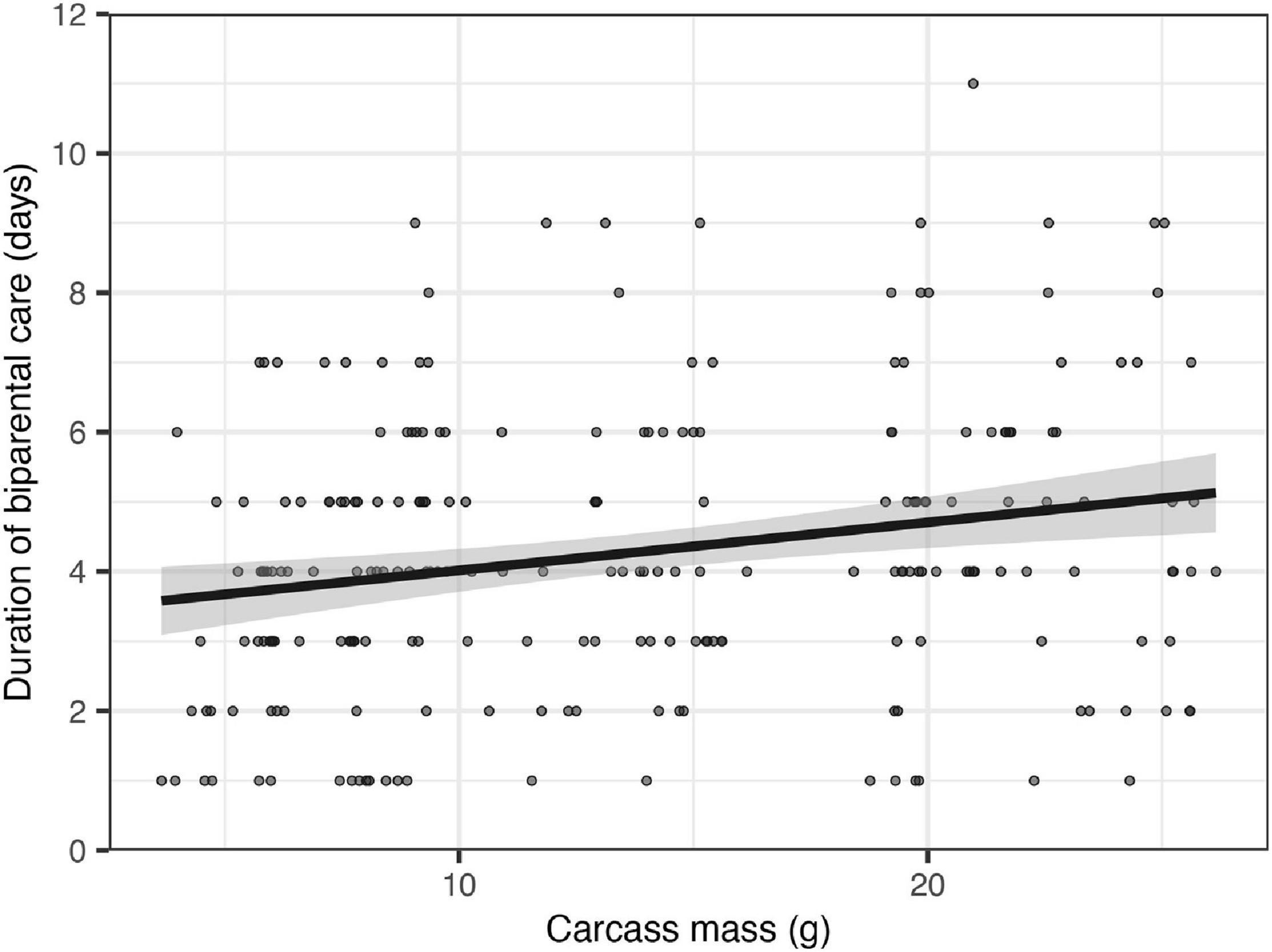
Figure 3. Effects of carcass mass on the duration of biparental care. Filled circles represent individual data point, the line represents linear regression lines and shaded ribbons the 95% confidence intervals.
Offspring Behaviour and Performance
There was no significant effect of carcass mass on the average time spent begging by individual larvae (Table 2). However, brood size at dispersal increased by approximately 2 larvae for each additional 10 g of carcass (Table 2 and Figure 4A; estimate = 0.016, SE = 0.006, z = 2.51, P = 0.012) and, for smaller carcasses (i.e., below 10 g), mean larval mass at dispersal increased by approximately 0.026 g for each additional 10 g of carcass (Table 2 and Figure 4B; estimate = 0.025, SE = 0.006, t = 4.00, P < 0.001). There were significant effects of both the quadratic (χ2 = 8.89, df = 1, P = 0.0028) and the cubic (χ2 = 5.52, df = 1, P = 0.018) terms of carcass mass on mean larval mass at dispersal. Thus, mean larval mass increased with carcass mass when carcasses were relatively small and plateaued as carcass mass approached the upper end of the range of carcasses used in our experiment (Figure 4B). In addition, the duration of biparental care had a positive effect on brood size at dispersal (χ2 = 5.91, df = 1, P = 0.015), increasing by approximately 0.8 larvae for each additional day of biparental care. The duration of biparental care had no effect on mean larval mass at dispersal (χ2 = 0.324, df = 1, P = 0.568). Including clutch size in the model of brood size at dispersal removed the significant effect of carcass mass (Table 2), suggesting that the effect of carcass mass on brood size at dispersal was driven by differences in the number of eggs laid on carcasses of different masses. Including or excluding clutch size in the model on mean larval mass did not change the effect of carcass mass (Table 2), suggesting that the effects of carcass mass on mean larval mass was independent of any potential effects due to the number of offspring in the brood.
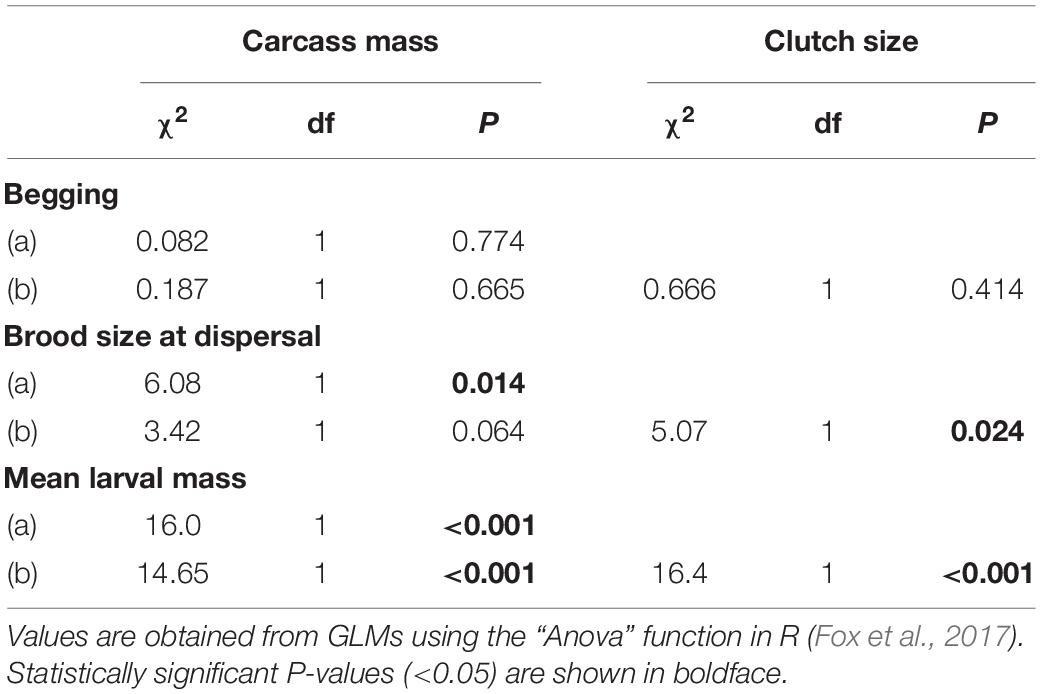
Table 2. Effects of carcass mass on larval begging, brood size at dispersal, and mean larval mass at dispersal when clutch size is excluded (a) and included (b) in the model.
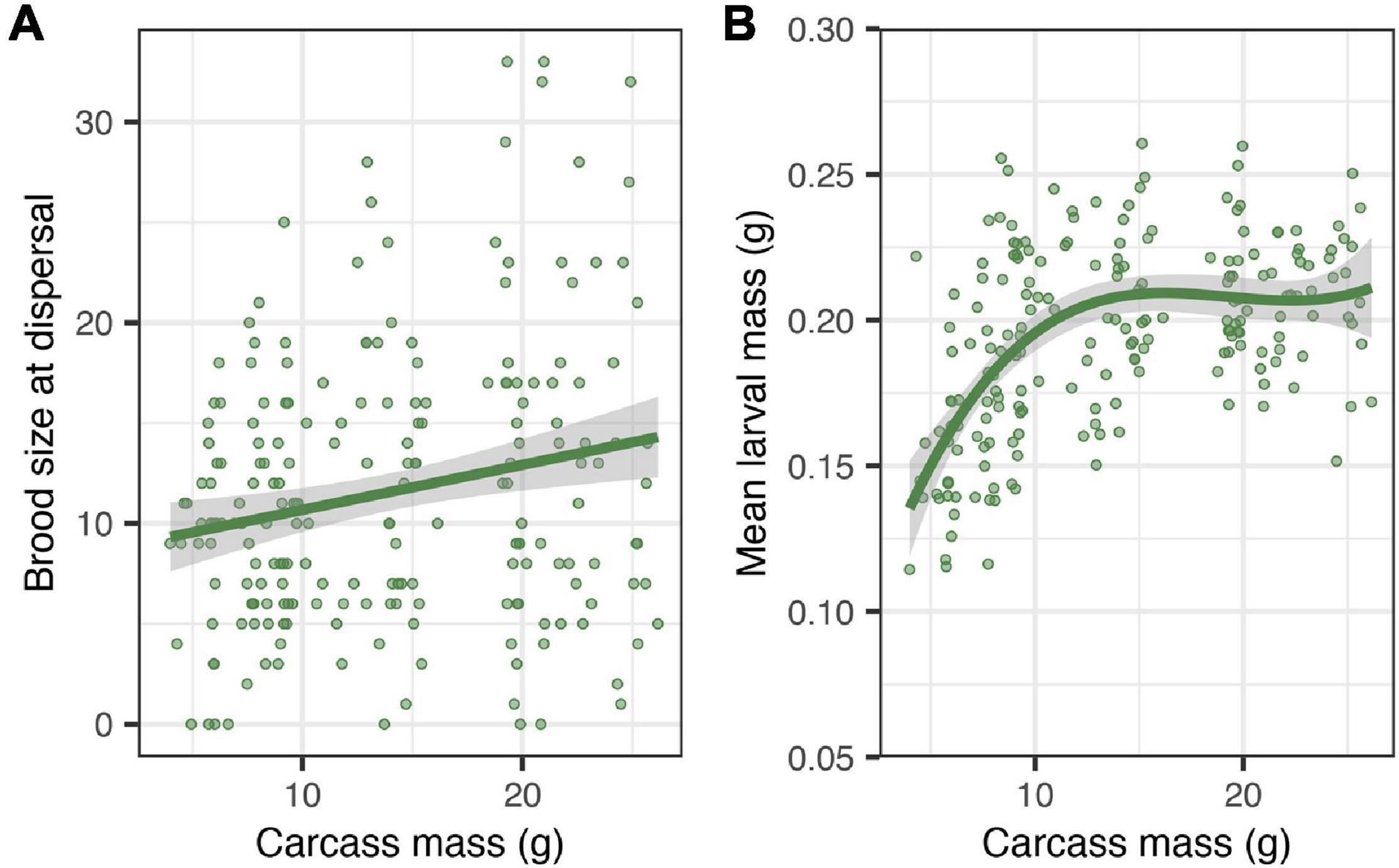
Figure 4. Effects of carcass mass on brood size at dispersal (A) and mean larval mass at dispersal (B). Filled circles represent individual data points; lines represent a linear regression line in panel (A) and a polynomial regression line in panel (B), and shaded ribbons the 95% confidence intervals.
Discussion
Here we show that a decrease in carcass mass was associated with more pronounced sex differences in both the duration of care and the time spent providing care, reflecting that males deserted the brood earlier and spent less time maintaining the carcass as carcass mass decreased. In contrast, females nearly always provided care until the larvae dispersed and spent a similar amount of time maintaining the carcass regardless of carcass mass. Furthermore, an increase in carcass mass was associated with a greater increase in weight gain by females than by males. Thus, variation in access to resources altered the magnitude of sex differences in parental care and parental weight change during breeding. We also found that an increase in carcass mass was associated with an increase in the duration of biparental care and in the benefits of care in terms of offspring survival, indicating a shift toward more cooperation between male and female parents as access to resources increased. Below we discuss the wider implications of our results for our understanding of how environmental conditions may drive the origin and maintenance of biparental care.
Our first main result was that there was a significant effect of the interaction between the sex of the focal parent and carcass mass on the duration of care and the amount of time spent maintaining the carcass on the day after hatching. These interaction effects reflected that males provided care for longer and spent more time maintaining the carcass as carcass mass increased, while carcass mass had no effect on the duration of care or time spent maintaining the carcass by females. These results are consistent with the findings on a related species of burying beetle (Kishida and Suzuki, 2010) and support our prediction that sex differences in parental care would be more pronounced as carcass mass decreased. Our results are consistent with prior work on N. vespilloides showing that females spend more time provisioning food to the brood (e.g., Smiseth et al., 2005; Georgiou-Shippi et al., 2018) and care for longer than males (Bartlett, 1988; Ford and Smiseth, 2016), and that males often adjust the amount of care they provide in response to variation in environmental conditions, whilst females tend to provide a similar amount of care regardless of such variation (Walling et al., 2008; Royle et al., 2014; Smiseth et al., 2005). These sex differences in parental care are thought to reflect that males can gain some reproductive success by mating away from a carcass whilst female require access to a carcass in order to reproduce (Müller et al., 2007). Thus, variation in access to resources may have a greater impact on the duration of male care because it increases their benefits of providing care relative to their benefits of deserting to mate with females away from a carcass (Ward et al., 2009).
We found that carcass mass had a greater positive effect on female weight gain than on male weight gain. This finding contrasts with our prediction that carcass mass would have a stronger impact on male weight change. Our prediction was based on the assumption that, if males provided care for longer on larger carcasses, this would give them more opportunities to consume from the carcass. Thus, our results contradict our initial assumption that sex differences in weight change would be linked to sex differences in parental care. This assumption is also contradicted by the finding that females gained more weight as carcass mass increased, even though females nearly always provided care until the larvae dispersed. Females gaining more weight as carcass mass increased suggests that females balance the personal benefits of consuming food from the carcass in terms of enhancing their own condition at the end of breeding against the costs of consuming food to the detriment of the larvae (Gray et al., 2018; Keppner et al., 2020). In this species, both the parents and the larvae feed from the carcass, and any increase in food consumption by a parent would therefore reduce the amount of food available to the other parent and the brood. Thus, females might restrict their own food consumption when breeding on smaller carcasses to avoid inflicting a cost to the larvae. On larger carcasses, where food is more plentiful, females may consume more food and put on more weight without inflicting such a cost to the larvae. However, it is unclear why this argument would only apply to female weight change. One potential explanation for why males seem to gain a similar amount of weight regardless of carcass mass is that males have a lower optimal body weight compared to females. Females may have a higher optimal body weight than males given that females must secure a carcass to reproduce, which means that they must fly in search of a carcass and compete with rival females. Gaining more weight might be beneficial given that flight is energetically costly and that heavier females tend to win more fights than lighter ones (Richardson et al., 2020). In contrast, males can attract and mate with females away from a carcass by emitting pheromones (Pukowski, 1933) and emitting pheromones is presumably less energetically costly than flying. Although carrion consumption might have a positive effect on male pheromone production and attractiveness (Chemnitz et al., 2017), a potential interpretation of results from the present study and others reporting greater body weight in females relative to males (e.g., Pilakouta et al., 2016; Paquet and Smiseth, 2017) is that males benefit less from putting on more weight than females. Alternatively, it could reflect greater energy expenditure by males increasing their effort in maintaining larger carcasses compared to smaller ones. This is because larger carcasses, which are heavier and have a greater surface area, potentially require greater effort to bury, prepare, and suppress bacterial and fungal growth from its surface (Xu and Suzuki, 2001). This is, however, unlikely to explain our results given that we found no evidence that males on larger carcasses consume more food. Nevertheless, we encourage future research to investigate this issue and examine the potential causes for sex differences in optimal body mass.
Our second main result was that the duration of biparental care increased with carcass mass. This result, together with the fact that males gained a similar amount of weight on smaller and larger carcasses, support our prediction that there was a shift toward more cooperation when parents had access to more resources. The rationale for our prediction was that the benefits of biparental cooperation would be greater on larger carcasses given that such carcasses are more valuable as a breeding resource to conspecific intruders, which may attempt to take over the carcass from the resident parents (Trumbo, 1991). If successful, such intruders would eliminate the original brood and use what is left of the carcass to rear their own brood. Furthermore, a study on the closely related N. orbicollis found that two parents are better able to protect the brood against conspecific intruders than single parents (Trumbo, 1991). Given that larger carcasses are subject to more intense competition than smaller ones (Wilson and Fudge, 1984; Robertson, 1993), it seems likely that the benefits to the male from assisting the female (and to the female from accepting assistance from the male) in terms of enhanced offspring survival would be greater as carcass mass increases. Our results contrast with comparative studies on birds, which have found that biparental cooperation was less common in species that breed in environments where there is greater availability of resources (Crook, 1963; Leisler et al., 2002; Barve and La Sorte, 2016). In altricial birds, greater access to food may reduce the benefits of biparental cooperation given that the female is more likely to be able to provision sufficient food for the brood on her own when food is plentiful as compared to when it is scarce. Biparental cooperation over food provisioning may be particularly important in altricial birds because parents must provide a constant supply of food from the surrounding environment. Thus, in altricial birds, the benefits of the male assisting the female may be greater when food is scarce. In contrast, biparental cooperation over food provisioning may be less important in burying beetles of the genus Nicrophorus. The reason for this is that these beetles breed on a fixed resource (i.e., a vertebrate carcass), which means that the supply of food will be limited by the size of the carcass rather than by the number of caring parents.
Our final results were that parents produced larvae with a greater mean mass when breeding on larger carcasses, whilst carcass mass had no effect on larval begging or brood size when controlling for clutch size. In contrast, the duration of biparental care had a positive effect on brood size only. The positive influence of carcass mass is consistent with previous findings reporting positive effects of carcass size on offspring growth and mass at dispersal (e.g., Xu and Suzuki, 2001; Andrews et al., 2017; Gray et al., 2018) but no effect on larval begging (Smiseth and Moore, 2002; Sieber et al., 2017). Such positive effects on offspring performance are likely to reflect that larvae simply have access to more food when self-feeding from the carcass, rather than an increase in the amount of care provided by the male. This is because the carcass represents the sole source of food for the larvae, and larvae may run out of food earlier on a smaller carcass than on a larger one. Moreover, prior work suggests that male care has no detectable effects on offspring growth and survival under laboratory conditions (Smiseth et al., 2005; Ratz et al., 2018), and may even have detrimental effects on females (Boncoraglio and Kilner, 2012). Our finding that the duration of biparental care had a positive effect on brood size, even when accounting for potential initial differences in clutch size, suggests that larvae cared for by two parents had a higher survival than larvae cared for by a single parent (Pilakouta et al., 2018). Taken together, these findings reveal that greater carcass mass can have positive effects on offspring performance through multiple mechanisms: (1) increasing the amount of food available to larvae, which enhances larval growth; and (2) increasing the duration of biparental care, which enhances larval survival.
In summary, we found that greater access to food reduced sex differences in parental care and shifted the balance toward more cooperation between parents. Overall, our findings stress the importance that environmental conditions, such as access to resources, play in determining the magnitude of any sex differences in parental behaviour, as well as determining the balance between cooperation and conflict over care. This is perhaps not surprising given that resource availability has long been recognised as a crucial environmental condition driving the emergence and maintenance of parental care in general (Tallamy and Wood, 1986; Klug et al., 2012). However, less consideration has been given to the role that resource availability plays as an environmental driver of the evolution of biparental care. Our findings also highlight the link between the magnitude of sex differences in care and shifts in the balance between cooperation and conflict. Such a link seems likely to emerge whenever variation in environmental conditions is associated with a greater reduction in the duration of care by parents of one sex as we report in our study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
TR, JR, and PS conceived and designed the experiments. KK, LL, and TR collected the data. TR analysed the data. TR and PS wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
TR was supported by the Darwin Trust of Edinburgh.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the City of Edinburgh Natural Heritage Service for permission to collect beetles in their reserve at the Blackford Hill Local Nature Reserve. We thank Lilia Galvez for assistance with maintaining the laboratory population. We are grateful to Jarrod Hadfield and Trine Bilde for thoughtful input on an earlier version of this manuscript.
References
Andrews, C. P., Kruuk, L. E. B., and Smiseth, P. T. (2017). Evolution of elaborate parental care: phenotypic and genetic correlations between parent and offspring traits. Behav. Ecol. 28, 39–48. doi: 10.1093/beheco/arw129
Balshine, S. (2012). “Patterns of parental care in vertebrates,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford: Oxford University Press), 62–80. doi: 10.1093/acprof:oso/9780199692576.003.0004
Bartlett, J. (1988). Male mating success and paternal care in Nicrophorus vespilloides (Coleoptera: Silphidae). Behav. Ecol. Sociobiol. 23, 297–303. doi: 10.1007/bf00300576
Bartlett, J., and Ashworth, C. M. (1988). Brood size and fitness in Nicrophorus vespilloides (Coleoptera: Silphidae). Behav. Ecol. Sociobiol. 22, 429–434. doi: 10.1007/bf00294981
Barve, S., and La Sorte, F. A. (2016). Fruiting season length restricts global distribution of female-only parental care in frugivorous passerine birds. PLoS One 11:e0154871. doi: 10.1371/journal.pone.0154871
Billman, E. J., Creighton, J. C., and Belk, M. C. (2014). Prior experience affects allocation to current reproduction in a burying beetle. Behav. Ecol. 25, 813–818. doi: 10.1093/beheco/aru051
Boncoraglio, G., and Kilner, R. M. (2012). Female burying beetles benefit from male desertion: sexual conflict and counter-adaptation over parental investment. PLoS One 7:e31713. doi: 10.1371/journal.pone.0031713
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/rj-2017-066
Chase, I. D. (1980). Cooperative and noncooperative behavior in animals. Am. Nat. 115, 827–857. doi: 10.1086/283603
Chemnitz, J., Bagrii, N., Ayasse, M., and Steiger, S. (2017). Staying with the young enhances the fathers’ attractiveness in burying beetles. Evolution 71, 985–994. doi: 10.1111/evo.13194
Cockburn, A. (2006). Prevalence of different modes of parental care in birds. Proc. R. Soc. Lond. B 273, 1375–1383. doi: 10.1098/rspb.2005.3458
Creighton, J. C., Heflin, N. D., and Belk, M. C. (2009). Cost of reproduction, resource quality, and terminal investment in a burying beetle. Am. Nat. 174, 673–784. doi: 10.1086/605963
De Gasperin, O., and Kilner, R. M. (2015). Interspecific interactions change the outcome of sexual conflict over prehatching parental investment in the burying beetle Nicrophorus vespilloides. Ecol. Evol. 5, 5552–5560. doi: 10.1002/ece3.1795
Eggert, A. K., and Müller, J. K. (1992). Joint breeding in female burying beetles. Behav. Ecol. Sociobiol. 31, 237–242.
Eggert, A.-K., Reinking, M., and Muüller, J. K. (1998). Parental care improves offspring survival and growth in burying beetles. Anim. Behav. 55, 97–107. doi: 10.1006/anbe.1997.0588
Eggert, A. K., and Sakaluk, S. K. (2000). Benefits of communal breeding in burying beetles: a field experiment. Ecol. Entomol. 25, 262–266. doi: 10.1046/j.1365-2311.2000.00262.x
Eldegard, K., and Sonerud, G. A. (2010). Experimental increase in food supply influences the outcome of within-family conflicts in Tengmalm’s owl. Behav. Ecol. Sociobiol. 64, 815–826. doi: 10.1007/s00265-009-0898-z
Ford, L. E., and Smiseth, P. T. (2016). Asynchronous hatching provides females with a means for increasing male care but incurs a cost by reducing offspring fitness. J. Evol. Biol. 29, 428–437. doi: 10.1111/jeb.12797
Fox, J., Weisberg, S., Adler, D., Bates, D., Baud-Bovy, G., Ellison, S., et al. (2017). Package ‘car’. Available online at: https://cran.r-project.org/web/packages/car/car.pdf (accessed February 26, 2021).
Georgiou-Shippi, A., Paquet, M., and Smiseth, P. T. (2018). Sex differences in parental defence against conspecific intruders in the burying beetle Nicrophorus vespilloides. Anim. Behav. 136, 21–29. doi: 10.1016/j.anbehav.2017.12.011
Gray, F. E., Richardson, J., Ratz, T., and Smiseth, P. T. (2018). No evidence for parent–offspring competition in the burying beetle Nicrophorus vespilloides. Behav. Ecol. 29, 1142–1149. doi: 10.1093/beheco/ary091
Grew, R., Ratz, T., Richardson, J., and Smiseth, P. T. (2019). Parental care buffers against effects of ambient temperature on offspring performance in an insect. Behav. Ecol. 30, 1443–1450. doi: 10.1093/beheco/arz100
Hartig, F. (2017). DHARMa: Residual Diagnostics for Hierarchical (multi-level/mixed) Regression Models. R package version 0.1, 5(5).
Hopwood, P. E., Moore, A. J., Tregenza, T., and Royle, N. J. (2015). Male burying beetles extend, not reduce, parental care duration when reproductive competition is high. J. Evol. Biol. 28, 1394–1402. doi: 10.1111/jeb.12664
Johnstone, R. A., and Savage, J. L. (2019). Conditional cooperation and turn-taking in parental care. Front. Ecol. Evol. 7:335. doi: 10.3389/fevo.2019.00335
Keenleyside, M. H. (1983). Mate desertion in relation to adult sex ratio in the biparental cichlid fish Herotilapia multispinosa. Anim. Behav. 31, 683–688. doi: 10.1016/s0003-3472(83)80223-1
Keppner, E. M., Ayasse, M., and Steiger, S. (2018). Manipulation of parental nutritional condition reveals competition among family members. J. Evol. Biol. 31, 822–832. doi: 10.1111/jeb.13266
Keppner, E. M., Ayasse, M., and Steiger, S. (2020). Contribution of males to brood care can compensate for their food consumption from a shared resource. Ecol. Evol. 10, 3535–3543. doi: 10.1002/ece3.6150
Kishida, R., and Suzuki, N. (2010). Effect of carcass size on feeding modes of larvae of Nicrophorus quadripunctatus Kraatz (Coleoptera: Silphidae). Psyche. 2010:206318.
Klug, H., Alonzo, S. H., and Bonsall, M. B. (2012). “Theoretical foundations of parental care,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford: Oxford University Press), 21–39.
Kokko, H., and Jennions, M. D. (2012). “Sex differences in parental care,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford: Oxford University Press), 101–116. doi: 10.1093/acprof:oso/9780199692576.003.0006
Lavery, R. J., and Keenleyside, M. H. (1990). Parental investment of a biparental cichlid fish, Cichlasoma nigrofasciatum, in relation to brood size and past investment. Animal behaviour 40, 1128–1137. doi: 10.1016/s0003-3472(05)80179-4
Leisler, B., Winkler, H., and Wink, M. (2002). Evolution of breeding systems in acrocephaline warblers. Auk 119, 379–390. doi: 10.1093/auk/119.2.379
Lessells, C. M. (2012). “Sexual conflict,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford: Oxford University Press), 150–170.
Lessells, C. M., and McNamara, J. M. (2012). Sexual conflict over parental investment in repeated bouts: negotiation reduces overall care. Proc. R. Soc. B Biol. Sci. 279, 1506–1514. doi: 10.1098/rspb.2011.1690
Magneville, C., Ratz, T., Richardson, J., and Smiseth, P. T. (2018). No evidence of sibling cooperation in the absence of parental care in Nicrophorus vespilloides. Evolution 72, 2803–2809. doi: 10.1111/evo.13622
Margulis, S. W. (1998). Relationships among parental inbreeding, parental behaviour and offspring viability in oldfield mice. Anim. Behav. 55, 427–438. doi: 10.1006/anbe.1997.0618
Monteith, K. M., Andrews, C., and Smiseth, P. T. (2012). Post-hatching parental care masks the effects of egg size on offspring fitness: a removal experiment on burying beetles. J. Evol. Biol. 25, 1815–1822. doi: 10.1111/j.1420-9101.2012.02567.x
Müller, J. K., Eggert, A.-K., and Furlkröger, E. (1990). Clutch size regulation in the burying beetle Necrophorus vespilloides Herbst (Coleoptera: Silphidae). J. Insect Behav. 3, 265–270. doi: 10.1007/bf01417917
Müller, J. K. (1987). Replacement of a lost clutch: a strategy for optimal resource utilization in Necrophorus vespilloides (Coleoptera: Silphidae). Ethology 76, 74–80. doi: 10.1111/j.1439-0310.1987.tb00673.x
Müller, J. K., Braunisch, V., Hwang, W., and Eggert, A.-K. (2007). Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 18, 196–203. doi: 10.1093/beheco/arl073
Paquet, M., and Smiseth, P. T. (2017). Females manipulate behavior of caring males via prenatal maternal effects. Proc. Natl. Acad. Sci. U.S.A. 114, 6800–6805.
Pilakouta, N., Hanlon, E. J., and Smiseth, P. T. (2018). Biparental care is more than the sum of its parts: experimental evidence for synergistic effects on offspring fitness. Proc. R. Soc. B Biol. Sci. 285:20180875. doi: 10.1098/rspb.2018.0875
Pilakouta, N., Richardson, J., and Smiseth, P. T. (2016). If you eat, I eat: resolution of sexual conflict over consumption from a shared resource. Anim. Behav. 111, 175–180. doi: 10.1016/j.anbehav.2015.10.016
Pukowski, E. (1933). Ökologische untersuchungen an Necrophorus F. Z. Morphol. Ökologie Tiere 27, 518–586.
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ratz, T., Castel, E., and Smiseth, P. T. (2018). Male assistance in parental care does not buffer against detrimental effects of maternal inbreeding on offspring. Front. Ecol. Evol. 6:196. doi: 10.3389/fevo.2018.00196
Ratz, T., and Smiseth, P. T. (2018). Flexible parents: joint effects of handicapping and brood size manipulation on female parental care in Nicrophorus vespilloides. J. Evol. Biol. 31, 646–656. doi: 10.1111/jeb.13254
Richardson, J., Stephens, J., and Smiseth, P. T. (2020). Increased allocation to reproduction reduces future competitive ability in a burying beetle. J. Anim. Ecol. 89, 1918–1926. doi: 10.1111/1365-2656.13242
Ripley, B., Venables, B., Bates, D. M., Hornik, K., Gebhardt, A., and Firth, D. (2017). Package “MASS.”.
Robertson, I. C. (1993). Nest intrusions, infanticide, and parental care in the burying beetle, Nicrophorus orbicollis (Coleoptera: Silphidae). J. Zool. 231, 583–593. doi: 10.1111/j.1469-7998.1993.tb01940.x
Royle, N. J., Russell, A. F., and Wilson, A. J. (2014). The evolution of flexible parenting. Science 345, 776–781. doi: 10.1126/science.1253294
Schwagmeyer, P. L., Bartlett, T. L., and Schwabl, H. G. (2008). Dynamics of house sparrow biparental care: what contexts trigger partial compensation? Ethology 114, 459–468. doi: 10.1111/j.1439-0310.2008.01480.x
Scott, M. P. (1998). The ecology and behavior of burying beetles. Annu. Rev. Entomol. 43, 595–618. doi: 10.1146/annurev.ento.43.1.595
Scott, M. P., and Traniello, J. F. A. (1990). Behavioural and ecological correlates of male and female parental and reproductive success in the burying beetle Nicrophorus orbicollis. Anim. Behav. 39, 274–283. doi: 10.1016/s0003-3472(05)80871-1
Shukla, S. P., Plata, C., Reichelt, M., Steiger, S., Heckel, D. G., Kaltenpoth, M., et al. (2018). Microbiome-assisted carrion preservation aids larval development in a burying beetle. Proc. Natl. Acad. Sci. U.S.A. 115, 11274–11279. doi: 10.1073/pnas.1812808115
Sieber, D. J., Paquet, M., and Smiseth, P. T. (2017). Joint effects of brood size and resource availability on sibling competition. Anim. Behav. 129, 25–30. doi: 10.1016/j.anbehav.2017.05.010
Smiseth, P. T., Andrews, C. P., Mattey, S. N., and Mooney, R. (2014). Phenotypic variation in resource acquisition influences trade-off between number and mass of offspring in a burying beetle. J. Zool. 293, 80–83. doi: 10.1111/jzo.12115
Smiseth, P. T., Darwell, C. T., and Moore, A. J. (2003). Partial begging: an empirical model for the early evolution of offspring signalling. Proc. R. Soc. B Biol. Sci. 270, 1773–1777. doi: 10.1098/rspb.2003.2444
Smiseth, P. T., Dawson, C., Varley, E., and Moore, A. J. (2005). How do caring parents respond to mate loss? Differential response by males and females. Anim. Behav. 69, 551–559. doi: 10.1016/j.anbehav.2004.06.004
Smiseth, P. T., Lennox, L., and Moore, A. J. (2007). Interaction between parental care and sibling competition: parents enhance offspring growth and exacerbate sibling competition. Evolution 61, 2331–2339. doi: 10.1111/j.1558-5646.2007.00192.x
Smiseth, P. T., and Moore, A. J. (2002). Does resource availability affect offspring begging and parental provisioning in a partially begging species? Anim. Behav. 63, 577–585. doi: 10.1006/anbe.2001.1944
Smiseth, P. T., and Moore, A. J. (2004). Behavioral dynamics between caring males and females in a beetle with facultative biparental care. Behav. Ecol. 15, 6216–6228.
Smiseth, P. T., Ward, R. J. S., and Moore, A. J. (2006). Asynchronous hatching in Nicrophorus vespilloides, an insect in which parents provide food for their offspring. Funct. Ecol. 20, 151–156. doi: 10.1111/j.1365-2435.2006.01072.x
Tallamy, D. W., and Wood, T. K. (1986). Convergence patterns in subsocial insects. Annu. Rev. Entomol. 31, 369–390. doi: 10.1146/annurev.en.31.010186.002101
Trivers, R. L. (1972). “Parental investment and sexual selection,” in Sexual Selection and the Descent of Man 1871–1971, ed. B. Campbell (London: Heinemann), 136–139. doi: 10.4324/9781315129266-7
Trumbo, S. T. (1991). Reproductive benefits and the duration of paternal care in a biparental burying beetle, Necrophorus orbicollis. Behaviour 117, 82–105. doi: 10.1163/156853991x00139
Trumbo, S. T. (1992). Monogamy to communal breeding: exploitation of a broad resource base by burying beetles (Nicrophorus). Ecol. Entomol. 17, 289–298. doi: 10.1111/j.1365-2311.1992.tb01060.x
Trumbo, S. T. (2006). Infanticide, sexual selection and task specialization in a biparental burying beetle. Anim. Behav. 72, 1159–1167. doi: 10.1016/j.anbehav.2006.05.004
Trumbo, S. T. (2007). Defending young biparentally: female risk-taking with and without a male in the burying beetle, Nicrophorus pustulatus. Behav. Ecol. Sociobiol. 61, 1717–1723. doi: 10.1007/s00265-007-0403-5
Trumbo, S. T. (2012). “Patterns of parental care in invertebrates,” in The Evolution of Parental Care, eds N. J. Royle, P. T. Smiseth, and M. Kölliker (Oxford: Oxford University Press), 81–100. doi: 10.1093/acprof:oso/9780199692576.003.0005
Vincze, O., Székely, T., Küpper, C., AlRashidi, M., Amat, J. A., Ticó, A. A., et al. (2013). Local environment but not genetic differentiation influences biparental care in ten plover populations. PLoS One 8:e60998. doi: 10.1371/journal.pone.0060998
Walling, C. A., Stamper, C. E., Smiseth, P. T., and Moore, A. J. (2008). The quantitative genetics of sex differences in parenting. Proc. Natl. Acad. Sci. U.S.A. 105, 18430–18435. doi: 10.1073/pnas.0803146105
Ward, R. J., Cotter, S. C., and Kilner, R. M. (2009). Current brood size and residual reproductive value predict offspring desertion in the burying beetle Nicrophorus vespilloides. Behav. Ecol. 20, 1274–1281. doi: 10.1093/beheco/arp132
West, H. E., and Capellini, I. (2016). Male care and life history traits in mammals. Nat. Commun. 7:11854.
Westneat, D. F., and Sargent, R. C. (1996). Sex and parenting: the effects of sexual conflict and parentage on parental strategies. Trends Ecol. Evol. 11, 87–91. doi: 10.1016/0169-5347(96)81049-4
Whittingham, L. A. (1989). An experimental study of paternal behavior in red-winged blackbirds. Behav. Ecol. Sociobiol. 25, 73–80. doi: 10.1007/bf00299713
Wilson, D. S., and Fudge, J. (1984). Burying beetles: intraspecific interactions and reproductive success in the field. Ecol. Entomol. 9, 195–203. doi: 10.1111/j.1365-2311.1984.tb00715.x
Keywords: behavioural plasticity, biparental cooperation, parental care, environmental variation, Nicrophorus vespilloides
Citation: Ratz T, Kremi K, Leissle L, Richardson J and Smiseth PT (2021) Access to Resources Shapes Sex Differences Between Caring Parents. Front. Ecol. Evol. 9:712425. doi: 10.3389/fevo.2021.712425
Received: 20 May 2021; Accepted: 29 June 2021;
Published: 20 July 2021.
Edited by:
Ákos Pogány, Eötvös Loránd University, HungaryReviewed by:
Kyle Benowitz, University of Arizona, United StatesSandra Steiger, University of Bayreuth, Germany
Copyright © 2021 Ratz, Kremi, Leissle, Richardson and Smiseth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tom Ratz, ratz.tom@uqam.com
 Tom Ratz
Tom Ratz Katerina Kremi2
Katerina Kremi2  Per T. Smiseth
Per T. Smiseth