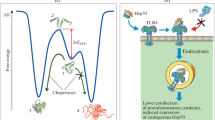
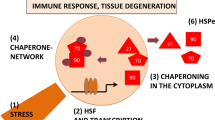
Abstract
This review describes a brief history of the discovery and studies in Russia and associated countries of the main stress protein (Hsp70) that plays important roles both in the normal function of the cell and body as well as under various stressful stimuli. Research on this protein at the Institute of Molecular Biology (Moscow) began with the elucidation of its adaptive functions at the cellular level and at the level of the whole organism. These studies examined the function of Hsp70 under normal and extreme conditions using a wide range of model and non-model animal species, from Leishmania and Drosophila to camels and humans. These analyses made it possible to elucidate the primary regulations in the evolution and function of heat shock (HS) genes in the studied organisms. Next, we studied the structure and characteristic features of heat shock genes and proteins in species with contrasting habitat temperatures. The systems of Hsp70 expression and isolation we developed using various research objects allowed us to proceed to study the protective properties of human recombinant Hsp70 in normal-aging animal models as well as animal models experiencing sepsis, Alzheimer’s disease, and stroke. The results obtained open the prospects of using recombinant Hsp70 for the treatment of various neuropathologies in humans. This review describes the logic and history of investigation of Hsp70 performed by one group of scientists from Engelhardt Institute of Molecular Biology, Russian Academy of Sciences. It was not the goal of this paper to give a comprehensive general picture of other similar studies carried out in Russia during this period.







Similar content being viewed by others
References
Asea (2008) A. 291 Hsp70: a chaperokine. In: Novartis Foundation symposium. Wiley Online Library, p 173
Astakhova LN, Zatsepina OG, Funikov SY, Zelentsova ES, Schostak NG, Orishchenko KE, Evgen’ev MB, Garbuz DG (2015) Activity of heat shock genes' promoters in thermally contrasting animal species. PLoS One 10(2):e0115536. https://doi.org/10.1371/journal.pone.0115536
Bobkova N, Guzhova I, Margulis B, Nesterova I, Medvinskaya N, Medvedinskaya N et al (2013) Dynamics of endogenous Hsp70 synthesis in the brain of olfactory bulbectomized mice. Cell Stress Chaperones 18(1):109–118. https://doi.org/10.1007/s12192-012-0359-x
Bobkova NV, Garbuz DG, Nesterova I, Medvinskaya N, Samokhin A, Alexandrova I, Yashin V, Karpov V, Kukharsky MS, Ninkina NN, Smirnov AA, Nudler E, Evgen'ev M (2014) Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer’s disease. J Alzheimers Dis 38(2):425–435. https://doi.org/10.3233/JAD-130779
Bobkova NV, Evgen'ev M, Garbuz DG, Kulikov AM, Morozov A, Samokhin A et al (2015) Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. Proc Natl Acad Sci U S A 112(52):16006–16011. https://doi.org/10.1073/pnas.1516131112
Demyanenko S, Nikul V, Rodkin S, Davletshin A, Evgen'ev MB, Garbuz DG (2021) Exogenous recombinant Hsp70 mediates neuroprotection after photothrombotic stroke. Cell Stress Chaperones 26(1):103–114. https://doi.org/10.1007/s12192-020-01159-0
Evgen’ev M, Scheinker V, Levin A (1987) Molecular mechanisms of adaptation to hyperthermia in eukaryotic organisms. I Heat shock protein synthesis pattern in cell cultures and caterpillars of two silk worm species Mol Biol (Rus) 21:484–494
Evgen’ev MB, Zatsepina OG, Garbuz D, Lerman DN, Velikodvorskaya V, Zelentsova E, Feder ME (2004) Evolution and arrangement of the hsp70 gene cluster in two closely related species of the virilis group of Drosophila. Chromosoma 113(5):223–232
Evgen'ev M, Kolchinski A, Levin A, Preobrazhenskaya O, Sarkisova E (1978) Heat-shock DNA homology in distantly related species of Drosophila. Chromosoma 68(4):357–365
Evgen'ev M, Levin A, Lozovskaya E (1979) The analysis of a temperature-sensitive (ts) mutation influencing the expression of heat shock-inducible genes in Drosophila melanogaster. Mol Gen Genet 176(2):275–280. https://doi.org/10.1007/BF00273222
Evgen'ev MB, Garbuz DG, Zatsepina OG (2014) Heat shock proteins and whole body adaptation to extreme environments. Springer
Evgen'ev MB, Krasnov GS, Nesterova IV, Garbuz DG, Karpov VL, Morozov AV et al (2017) Molecular mechanisms underlying neuroprotective effect of intranasal administration of human Hsp70 in mouse model of Alzheimer’s disease. J Alzheimers Dis 59(4):1415–1426. https://doi.org/10.3233/JAD-170398
Evgen'ev M, Bobkova N, Krasnov G, Garbuz D, Funikov S, Kudryavtseva A et al (2019) The effect of human HSP70 administration on a mouse model of Alzheimer’s disease strongly depends on transgenicity and age. J Alzheimers Dis 67(4):1391–1404. https://doi.org/10.3233/JAD-180987
Garbuz DG, Astakhova LN, Zatsepina OG, Arkhipova IR, Nudler E, Evgen'ev MB (2011a) Functional organization of hsp70 cluster in camel (Camelus dromedarius) and other mammals. PLoS One 6(11):e27205
Garbuz DG, Yushenova IA, Zatsepina OG, Przhiboro AA, Bettencourt BR, Evgen'ev MB (2011b) Organization and evolution of hsp70 clusters strikingly differ in two species of Stratiomyidae (Diptera) inhabiting thermally contrasting environments. BMC Evol Biol 11(1):1–17
Garbuz DG, Sverchinsky D, Davletshin A, Margulis BA, Mitkevich V, Kulikov AM, Evgen’ev MB (2019) The molecular chaperone Hsp70 from the thermotolerant Diptera species differs from the Drosophila paralog in its thermostability and higher refolding capacity at extreme temperatures. Cell Stress Chaperones 24(6):1163–1173
Gehring WJ, Wehner R (1995) Heat shock protein synthesis and thermotolerance in Cataglyphis, an ant from the Sahara desert. Proc Natl Acad Sci 92(7):2994–2998
Gong WJ, Golic KG (2004) Genomic deletions of the Drosophila melanogaster Hsp70 genes. Genetics 168(3):1467–1476. https://doi.org/10.1534/genetics.104.030874
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475(7356):324–332. https://doi.org/10.1038/nature10317
Hightower LE (1991) Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 66(2):191–197
Hunt C, Morimoto RI (1985) Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci 82(19):6455–6459
Karpov VL, Preobrazhenskaya OV, Mirzabekov AD (1984) Chromatin structure of hsp 70 genes, activated by heat shock: selective removal of histones from the coding region and their absence from the 5′ region. Cell 36(2):423–431
Kustanova GA, Murashev AN, Karpov VL, Margulis BA, Guzhova IV, Prokhorenko IR et al (2006) Exogenous heat shock protein 70 mediates sepsis manifestations and decreases the mortality rate in rats. Cell Stress Chaperones 11(3):276–286. https://doi.org/10.1379/csc-195r.1
Leak RK (2014) Heat shock proteins in neurodegenerative disorders and aging. Journal of cell communication and signaling 8(4):293–310
Lewis M, Helmsing PJ, Ashburner M (1975) Parallel changes in puffing activity and patterns of protein synthesis in salivary glands of Drosophila. Proc Natl Acad Sci 72(9):3604–3608
Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55(1):1151–1191
Lyashko VN, Vikulova VK, Chernicov VG, Ivanov VI, Ulmasov KA, Zatsepina OG, Evgen'ev MB (1994) Comparison of the heat shock response in ethnically and ecologically different human populations. Proc Natl Acad Sci 91(26):12492–12495
Mayer MP (2010) Gymnastics of molecular chaperones. Mol Cell 39(3):321–331
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12(24):3788–3796
Nesterova IV, Bobkova NV, Medvinskaya NI, Samokhin AN, Aleksandrova IY (2008) Morphofunctional state of neurons in the temporal cortex and hippocampus in relation to the level of spatial memory in rats after ablation of the olfactory bulbs. Neurosci Behav Physiol 38(4):349–353. https://doi.org/10.1007/s11055-008-0048-5
Norris CE, DiIorio PJ, Schultz RJ, Hightower LE (1995) Variation in heat shock proteins within tropical and desert species of poeciliid fishes. Mol Biol Evol 12(6):1048–1062
Parsell D, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–497
Ritossa F (1962) A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 18(12):571–573
Rozhkova E, Yurinskaya M, Zatsepina O, Garbuz D, Karpov V, Surkov S, Murashev A, Ostrov V, Margulis B, Evgen’ev M, Vinokurov M (2010) Exogenous mammalian extracellular HSP70 reduces endotoxin manifestations at the cellular and organism levels. Ann N Y Acad Sci 1197:94–107. https://doi.org/10.1111/j.1749-6632.2009.05375.x
Shilova VY, Zatsepina OG, Garbuz DG, Funikov SY, Zelentsova ES, Schostak NG, Kulikov AM, Evgen'ev MB (2018) Heat shock protein 70 from a thermotolerant Diptera species provides higher thermoresistance to Drosophila larvae than correspondent endogenous gene. Insect Mol Biol 27(1):61–72. https://doi.org/10.1111/imb.12339
Tissiéres A, Mitchell HK, Tracy UM (1974) Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol 84(3):389–398
Tret'iakova IV, Lezin GT, Markova EG, Evgen'ev MB, Mamon LA (2001) The sbr gene product in Drosophila melanogaster and its orthologs in yeast (Mex67p) and human (TAP). Genetika 37(6):725–736
Ulmasov KA, Shammakov S, Karaev K, Evgen'ev MB (1992) Heat shock proteins and thermoresistance in lizards. Proc Natl Acad Sci 89(5):1666–1670
Ulmasov HA, Karaev KK, Lyashko VN, Evgen'ev MB (1993) Heat-shock response in camel (Camelus dromedarius) blood cells and adaptation to hyperthermia. Comparative biochemistry and physiology. B, Comparative biochemistry 106(4):867–872
Ulmasov K, Zatsepina O, Evgen'ev M, Molodtsov V (1999) Natural body temperature and kinetics of heat-shock protein synthesis in the toad-headed agamid lizard Phrynocephalus interscapularis. Amphibia-Reptilia 20(1):1–9
Vinokurov M, Ostrov V, Yurinskaya M, Garbuz D, Murashev A, Antonova O, Evgen’ev M (2012) Recombinant human Hsp70 protects against lipoteichoic acid-induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones 17(1):89–101. https://doi.org/10.1007/s12192-011-0288-0
White CN, Hightower LE, Schultz R (1994) Variation in heat-shock proteins among species of desert fishes (Poeciliidae, Poeciliopsis). Mol Biol Evol 11(1):106–119
Wu C (1995) Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol 11(1):441–469
Yurinskaya M, Zatsepina OG, Vinokurov MG, Bobkova NV, Garbuz DG, Morozov AV, Kulikova D, Mitkevich V, Makarov A, Funikov S, Evgen’ev M (2015) The fate of exogenous human HSP70 introduced into animal cells by different means. Curr Drug Deliv 12(5):524–532. https://doi.org/10.2174/1567201812666150724094207
Yurinskaya MM, Garbuz DG, Evgen'ev MB, Vinokurov MG (2020) Exogenous HSP70 and signaling pathways involved in the inhibition of LPS-induced neurotoxicity of neuroblastoma cells. Mol Biol (Mosk) 54(1):128–136. https://doi.org/10.31857/S0026898420010164
Acknowledgements
Dozens of people from various institutes and universities in Russia and other countries participated in this long-term work to varying degrees. I would especially like to mention the role of the late Khayet Ulmasov, who conducted the primary field research in Turkmenistan, as well as my long-term collaborators Olga Zatsepina and David Garbuz, who participated in the study of Hsps during the main stages. I would also like to thank Natalia Bobkova, together with whom all the experiments on ageing and the study of AD models were performed, as well as Maxim Vinokurov and Marina Yurinskaya, who performed all the work with cell cultures. At many stages of the work, we discussed our results with Boris Margulis and Irina Guzhova, who are our co-authors on a number of articles. The author expresses his deep gratitude to Dr. Lawrence Hightower and Dr. Robert Tanguay for reading the MS and many useful suggestions and corrections.
Funding
This work was supported by Russian Science Foundation Grant 17-74-30030.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Evgen’ev, M.B. Heat shock proteins: a history of study in Russia. Cell Stress and Chaperones 26, 617–627 (2021). https://doi.org/10.1007/s12192-021-01219-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-021-01219-z