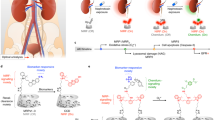
Abstract
In nephrology, differential diagnosis or assessment of disease activity largely relies on the analysis of glomerular filtration rate, urinary sediment, proteinuria and tissue obtained through invasive kidney biopsies. However, currently available non-invasive functional parameters, and most serum and urine biomarkers, cannot capture intrarenal molecular disease processes specifically. Moreover, although histopathological analyses of kidney biopsy samples enable the visualization of pathological morphological and molecular alterations, they only provide information about a small part of the kidney and do not allow longitudinal monitoring. These limitations not only hinder understanding of the dynamics of specific disease processes in the kidney, but also limit the targeting of treatments to active phases of disease and the development of novel targeted therapies. Molecular imaging enables non-invasive and quantitative assessment of physiological or pathological processes by combining imaging technologies with specific molecular probes. Here, we discuss current preclinical and clinical molecular imaging approaches in nephrology. Non-invasive visualization of the kidneys through molecular imaging can be used to detect and longitudinally monitor disease activity and can therefore provide companion diagnostics to guide clinical trials, as well as the safe and effective use of drugs.
Key points
-
Today, nephrology relies on the analysis of glomerular filtration rate, urinary sediment, proteinuria and invasive kidney biopsies to assess disease activity; molecular imaging is a more specific non-invasive approach that visualizes pathological processes within the kidneys with high accuracy.
-
18F-fluorodeoxyglucose–PET is currently the most commonly used approach of molecular kidney imaging, although it does not reflect a specific disease or pathway.
-
Most clinical trials of molecular kidney imaging are performed in patients with renal cell carcinomas and promising targets include carbonic anhydrase 9 and prostate-specific membrane antigen.
-
Molecular imaging of the kidneys is challenging because it is a major elimination organ and non-specific probe uptake can be high; however, preclinical experiments and early clinical studies have identified targets and established imaging protocols for molecular imaging of acute and chronic kidney diseases, with a focus on acute epithelial or endothelial cell injury, inflammation or fibrosis.
-
Similar to oncology, molecular kidney imaging might improve disease staging, prognostication, monitoring of treatment responses and patient management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
05 July 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41581-021-00464-w
References
Thakur, M. L., Lentle, B. C. & SNM; Radiological Society of North America (RSNA). Joint SNM/RSNA Molecular Imaging Summit Statement. J. Nucl. Med. 46, 11N–13N, 42N (2005).
Mankoff, D. A. A definition of molecular imaging. J. Nucl. Med. 48, 18N, 21N (2007).
Ehlerding, E. B., England, C. G., McNeel, D. G. & Cai, W. Molecular imaging of immunotherapy targets in cancer. J. Nucl. Med. 57, 1487–1492 (2016).
Mankoff, D. A., Farwell, M. D., Clark, A. S. & Pryma, D. A. Making molecular imaging a clinical tool for precision oncology: a review. JAMA Oncol. 3, 695–701 (2017).
Allali, G. et al. Brain imaging of locomotion in neurological conditions. Neurophysiol. Clin. 48, 337–359 (2018).
Chen, I. Y. & Wu, J. C. Cardiovascular molecular imaging: focus on clinical translation. Circulation 123, 425–443 (2011).
Farber, G. et al. The future of cardiac molecular imaging. Semin. Nucl. Med. 50, 367–385 (2020).
Taylor, A. T. Radionuclides in nephrourology, part 1: radiopharmaceuticals, quality control, and quantitative indices. J. Nucl. Med. 55, 608–615 (2014).
Taylor, A. T., Lipowska, M. & Cai, H. 99mTc(CO)3(NTA) and 131I-OIH: comparable plasma clearances in patients with chronic kidney disease. J. Nucl. Med. 54, 578–584 (2013).
Jaksic, E. et al. Clinical investigations of 99mTc-p-aminohippuric acid as a new renal agent. Nucl. Med. Commun. 30, 76–81 (2009).
Nguyen, D. L. et al. Reproducibility of differential renal function measurement using technetium-99m-ethylenedicysteine dynamic renal scintigraphy: a French prospective multicentre study. Nucl. Med. Commun. 39, 10–15 (2018).
Stoffel, M. et al. Evaluation of technetium-99m-L,L-EC in renal transplant recipients: a comparative study with technetium-99m-MAG3 and iodine-125-OIH. J. Nucl. Med. 35, 1951–1958 (1994).
Weyer, K. et al. Renal uptake of 99mTc-dimercaptosuccinic acid is dependent on normal proximal tubule receptor-mediated endocytosis. J. Nucl. Med. 54, 159–165 (2013).
Lee, B. H. et al. Decreased renal uptake of (99m)Tc-DMSA in patients with tubular proteinuria. Pediatr. Nephrol. 24, 2211–2216 (2009).
Bobot, M. et al. Renal SPECT/CT with 99mTc-dimercaptosuccinic acid is a non-invasive predictive marker for the development of interstitial fibrosis in a rat model of renal insufficiency. Nephrol. Dial. Transpl. 36, 804–810 (2020).
Fatemikia, H. et al. Comparison of 99mTc-DMSA renal scintigraphy with biochemical and histopathological findings in animal models of acute kidney injury. Mol. Cell Biochem. 434, 163–169 (2017).
Hitzel, A. et al. Quantitative analysis of 99mTc-DMSA during acute pyelonephritis for prediction of long-term renal scarring. J. Nucl. Med. 45, 285–289 (2004).
Stieger, B., Unadkat, J. D., Prasad, B., Langer, O. & Gali, H. Role of (drug) transporters in imaging in health and disease. Drug Metab. Dispos. 42, 2007–2015 (2014).
Santos, A. I. et al. Interobserver agreement on cortical tracer transit in 99mTc-MAG3 renography applied to congenital hydronephrosis. Nucl. Med. Commun. 38, 124–128 (2017).
Funahashi, Y. et al. Effect of warm ischemia on renal function during partial nephrectomy: assessment with new 99mTc-mercaptoacetyltriglycine scintigraphy parameter. Urology 79, 160–164 (2012).
Pathuri, G. et al. Evaluation of (99m)Tc-probestin SPECT as a novel technique for noninvasive imaging of kidney aminopeptidase N expression. Mol. Pharm. 11, 2948–2953 (2014).
Longo, D. L., Busato, A., Lanzardo, S., Antico, F. & Aime, S. Imaging the pH evolution of an acute kidney injury model by means of iopamidol, a MRI-CEST pH-responsive contrast agent. Magn. Reson. Med. 70, 859–864 (2013).
Irrera, P., Consolino, L., Cutrin, J. C., Zollner, F. G. & Longo, D. L. Dual assessment of kidney perfusion and pH by exploiting a dynamic CEST-MRI approach in an acute kidney ischemia-reperfusion injury murine model. NMR Biomed. 33, e4287 (2020).
Clatworthy, M. R. et al. Magnetic resonance imaging with hyperpolarized [1,4-C-13(2)]fumarate allows detection of early renal acute tubular necrosis. Proc. Natl Acad. Sci. USA 109, 13374–13379 (2012).
Nielsen, P. M. et al. Fumarase activity: an in vivo and in vitro biomarker for acute kidney injury. Sci. Rep. 7, 40812 (2017).
Gallagher, F. A. et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Natl Acad. Sci. USA 106, 19801–19806 (2009).
Huang, J., Li, J., Lyu, Y., Miao, Q. & Pu, K. Molecular optical imaging probes for early diagnosis of drug-induced acute kidney injury. Nat. Mater. 18, 1133–1143 (2019).
Allison, S. J. A molecular imaging approach for the early, real-time diagnosis of acute kidney injury. Nat. Rev. Nephrol. 15, 458 (2019).
Akhtar, A. M. et al. In vivo quantification of VCAM-1 expression in renal ischemia reperfusion injury using non-invasive magnetic resonance molecular imaging. PLoS ONE 5, e12800 (2010).
Hoyt, K. et al. Molecular ultrasound imaging of tissue inflammation using an animal model of acute kidney injury. Mol. Imaging Biol. 17, 786–792 (2015).
Boesen, E. I., Crislip, G. R. & Sullivan, J. C. Use of ultrasound to assess renal reperfusion and P-selectin expression following unilateral renal ischemia. Am. J. Physiol. Renal Physiol. 303, F1333–1340 (2012).
Andonian, S., Coulthard, T., Smith, A. D., Singhal, P. S. & Lee, B. R. Real-time quantitation of renal ischemia using targeted microbubbles: in-vivo measurement of P-selectin expression. J. Endourol. 23, 373–378 (2009).
Atukorale, P. U., Covarrubias, G., Bauer, L. & Karathanasis, E. Vascular targeting of nanoparticles for molecular imaging of diseased endothelium. Adv. Drug Deliv. Rev. 113, 141–156 (2017).
Guler, R., Svedmark, S. F., Abouzayed, A., Orlova, A. & Lofblom, J. Increasing thermal stability and improving biodistribution of VEGFR2-binding affibody molecules by a combination of in silico and directed evolution approaches. Sci. Rep. 10, 18148 (2020).
Wan, C. H., Tseng, J. R., Lee, M. H., Yang, L. Y. & Yen, T. C. Clinical utility of FDG PET/CT in acute complicated pyelonephritis-results from an observational study. Eur. J. Nucl. Med. Mol. Imaging 45, 462–470 (2018).
Pijl, J. P., Glaudemans, A., Slart, R. & Kwee, T. C. 18)F-FDG PET/CT in autosomal dominant polycystic kidney disease patients with suspected cyst infection. J. Nucl. Med. 59, 1734–1741 (2018).
Tseng, J. R. et al. Clinical usefulness of 18F-FDG PET/CT for the detection of infections of unknown origin in patients undergoing maintenance hemodialysis. J. Nucl. Med. 56, 681–687 (2015).
George, E. A., Codd, J. E., Newton, W. T., Haibach, H. & Donati, R. M. Comparative evaluation of renal transplant rejection with radioiodinated fibrinogen 99mTc-sulfur collid, and 67Ga-citrate. J. Nucl. Med. 17, 175–180 (1976).
Grabner, A. et al. Renal contrast-enhanced sonography findings in a model of acute cellular allograft rejection. Am. J. Transpl. 16, 1612–1619 (2016).
Grabner, A. et al. Noninvasive imaging of acute renal allograft rejection by ultrasound detection of microbubbles targeted to T-lymphocytes in rats. Ultraschall Med. 37, 82–91 (2016).
Martins, F. P., Souza, S. A., Goncalves, R. T., Fonseca, L. M. & Gutfilen, B. Preliminary results of [99mTc]OKT3 scintigraphy to evaluate acute rejection in renal transplants. Transpl. Proc. 36, 2664–2667 (2004).
Derlin, T. et al. Integrating MRI and chemokine receptor CXCR4-targeted PET for detection of leukocyte infiltration in complicated urinary tract infections after kidney transplantation. J. Nucl. Med. 58, 1831–1837 (2017).
Sargsyan, S. A. et al. Detection of glomerular complement C3 fragments by magnetic resonance imaging in murine lupus nephritis. Kidney Int. 81, 152–159 (2012).
Serkova, N. J. et al. Renal inflammation: targeted iron oxide nanoparticles for molecular MR imaging in mice. Radiology 255, 517–526 (2010).
Huang, Q. et al. C5b-9-targeted molecular MR imaging in rats with Heymann nephritis: a new approach in the evaluation of nephrotic syndrome. PLoS ONE 10, e0121244 (2015).
Smith, R. J. H. et al. C3 glomerulopathy — understanding a rare complement-driven renal disease. Nat. Rev. Nephrol. 15, 129–143 (2019).
Hanssen, O. et al. Non-invasive approaches in the diagnosis of acute rejection in kidney transplant recipients. Part I. In vivo imaging methods. Clin. Kidney J. 10, 97–105 (2017).
Lovinfosse, P. et al. Fluorodeoxyglucose F18 positron emission tomography coupled with computed tomography in suspected acute renal allograft rejection. Am. J. Transpl. 16, 310–316 (2016).
Even-Sapir, E. et al. Kidney allografts and remaining contralateral donor kidneys before and after transplantation: assessment by quantitative 99mTc-DMSA SPECT. J. Nucl. Med. 43, 584–588 (2002).
Bajen, M. T. et al. MAG3 renogram deconvolution in kidney transplantation: utility of the measurement of initial tracer uptake. J. Nucl. Med. 38, 1295–1299 (1997).
Benjamens, S. et al. Limited clinical value of two consecutive post-transplant renal scintigraphy procedures. Eur. Radiol. 30, 452–460 (2020).
Erbas, B. Peri- and postsurgical evaluations of renal transplant. Semin. Nucl. Med. 47, 647–659 (2017).
George, E. A., Codd, J. E., Newton, W. T. & Donati, R. M. 67Ga citrate in renal allograft rejection. Radiology 117, 731–733 (1975).
Solaric-George, E. A., Fletcher, J. W., Newton, W. T., Henry, R. E. & Donati, R. M. Renal accumulation of 99mTc sulfur colloid in transplant rejection. Radiology 111, 465–466 (1974).
George, E. A., Codd, J. E., Newton, W. T., Henry, R. E. & Donati, R. M. Further evaluation of 99mTc sulfur colloid accumulation in rejecting renal transplants in man and a canine model. Radiology 116, 121–126 (1975).
Liao, T. et al. Noninvasive quantification of intrarenal allograft C4d deposition with targeted ultrasound imaging. Am. J. Transpl. 19, 259–268 (2019).
Martin-Comin, J. Kidney graft rejection studies with labeled platelets and lymphocytes. Int. J. Rad. Appl. Instrum. B 13, 173–181 (1986).
Lopes de Souza, S. A. et al. Diagnosis of renal allograft rejection and acute tubular necrosis by 99mTc-mononuclear leukocyte imaging. Transpl. Proc. 36, 2997–3001 (2004).
Szablewski, L. Distribution of glucose transporters in renal diseases. J. Biomed. Sci. 24, 64 (2017).
Neuen, B. L. et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 7, 845–854 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
Rasul, S. et al. Response evaluation of SGLT2 inhibitor therapy in patients with type 2 diabetes mellitus using 18F-FDG PET/MRI. BMJ Open Diabetes Res. Care 8, e001135 (2020).
Sala-Rabanal, M. et al. Revisiting the physiological roles of SGLTs and GLUTs using positron emission tomography in mice. J. Physiol. 594, 4425–4438 (2016).
Mitsuoka, K. et al. Functional imaging of pharmacological action of SGLT2 inhibitor ipragliflozin via PET imaging using 11C-MDG. Pharmacol. Res. Perspect. 4, e00244 (2016).
Laustsen, C. et al. Hyperpolarized [1,4-13C]fumarate imaging detects microvascular complications and hypoxia mediated cell death in diabetic nephropathy. Sci. Rep. 10, 9650 (2020).
Qin, Z. et al. Molecular imaging of the glomerulus via mesangial cell uptake of radiolabeled tilmanocept. J. Nucl. Med. 60, 1325–1332 (2019).
Webster, A. C., Nagler, E. V., Morton, R. L. & Masson, P. Chronic kidney disease. Lancet 389, 1238–1252 (2017).
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Ku, E., Lee, B. J., Wei, J. & Weir, M. R. Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 74, 120–131 (2019).
Ismail, B. et al. Decreased renal AT1 receptor binding in rats after subtotal nephrectomy: PET study with [18F]FPyKYNE-losartan. EJNMMI Res. 6, 55 (2016).
Ismail, B. et al. Treatment with enalapril and not diltiazem ameliorated progression of chronic kidney disease in rats, and normalized renal AT1 receptor expression as measured with PET imaging. PLoS ONE 12, e0177451 (2017).
Baues, M. et al. Fibrosis imaging: current concepts and future directions. Adv. Drug Deliv. Rev. 121, 9–26 (2017).
van den Borne, S. W. et al. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J. Am. Coll. Cardiol. 52, 2017–2028 (2008).
Chen, D. L., Schiebler, M. L., Goo, J. M. & van Beek, E. J. R. PET imaging approaches for inflammatory lung diseases: current concepts and future directions. Eur. J. Radiol. 86, 371–376 (2017).
Makowski, M. R. et al. Assessment of atherosclerotic plaque burden with an elastin-specific magnetic resonance contrast agent. Nat. Med. 17, 383–388 (2011).
Ehling, J. et al. Elastin-based molecular MRI of liver fibrosis. Hepatology 58, 1517–1518 (2013).
Sun, Q. et al. Elastin imaging enables noninvasive staging and treatment monitoring of kidney fibrosis. Sci. Transl Med. 1, eaat4865 (2019).
Sanders, H. M. et al. The binding of CNA35 contrast agents to collagen fibrils. Chem. Commun. 47, 1503–1505 (2011).
Megens, R. T. et al. Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Mol. Imaging 6, 247–260 (2007).
Baues, M. et al. A collagen-binding protein enables molecular imaging of kidney fibrosis in vivo. Kidney Int. 97, 609–614 (2020).
Klinkhammer, B. M., Goldschmeding, R., Floege, J. & Boor, P. Treatment of renal fibrosis-turning challenges into opportunities. Adv. Chronic Kidney Dis. 24, 117–129 (2017).
Ak, I. & Can, C. F-18 FDG PET in detecting renal cell carcinoma. Acta Radiol. 46, 895–899 (2005).
Khandani, A. H., Cowey, C. L., Moore, D. T., Gohil, H. & Rathmell, W. K. Primary renal cell carcinoma: relationship between 18F-FDG uptake and response to neoadjuvant sorafenib. Nucl. Med. Commun. 33, 967–973 (2012).
Tabei, T. et al. Early assessment with 18F-2-fluoro-2-deoxyglucose positron emission tomography/computed tomography to predict short-term outcome in clear cell renal carcinoma treated with nivolumab. BMC Cancer 19, 298 (2019).
Nakaigawa, N. et al. FDG PET/CT as a prognostic biomarker in the era of molecular-targeting therapies: max SUVmax predicts survival of patients with advanced renal cell carcinoma. BMC Cancer 16, 67 (2016).
Minamimoto, R., Barkhodari, A., Harshman, L., Srinivas, S. & Quon, A. Prognostic value of quantitative metabolic metrics on baseline pre-sunitinib FDG PET/CT in advanced renal cell carcinoma. PLoS ONE 11, e0153321 (2016).
Vercellino, L. et al. 18F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: preliminary study. Cancer Biother Radiopharm. 24, 137–144 (2009).
Kelly-Morland, C. et al. Evaluation of treatment response and resistance in metastatic renal cell cancer (mRCC) using integrated 18F-Fluorodeoxyglucose (18F-FDG) positron emission tomography/magnetic resonance imaging (PET/MRI); The REMAP study. BMC Cancer 17, 392 (2017).
Chen, J. L. et al. FDG-PET as a predictive biomarker for therapy with everolimus in metastatic renal cell cancer. Cancer Med. 2, 545–552 (2013).
Fendler, W. P. et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur. J. Nucl. Med. Mol. Imaging 44, 1014–1024 (2017).
Kratochwil, C. et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 46, 2536–2544 (2019).
Beheshti, M. et al. Multiphasic 68Ga-PSMA PET/CT in the detection of early recurrence in prostate cancer patients with a PSA level of less than 1 ng/mL: a prospective study of 135 patients. J. Nucl. Med. 61, 1484–1490 (2020).
Endepols, H. et al. In vivo molecular imaging of glutamate carboxypeptidase II expression in re-endothelialisation after percutaneous balloon denudation in a rat model. Sci. Rep. 8, 7411 (2018).
Whitaker, H. C. et al. N-acetyl-L-aspartyl-L-glutamate peptidase-like 2 is overexpressed in cancer and promotes a pro-migratory and pro-metastatic phenotype. Oncogene 33, 5274–5287 (2014).
Morgenroth, A. et al. Targeting of prostate-specific membrane antigen for radio-ligand therapy of triple-negative breast cancer. Breast Cancer Res. 21, 116 (2019).
Siva, S. et al. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat. Rev. Urol. 17, 107–118 (2020).
Rowe, S. P. et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 29, 877–882 (2015).
Chen, Y. et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pen tanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin. Cancer Res. 17, 7645–7653 (2011).
Meyer, A. R. et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 33, 617–623 (2019).
Yin, Y. et al. Inconsistent detection of sites of metastatic non-clear cell renal cell carcinoma with PSMA-targeted [18F]DCFPyL PET/CT. Mol. Imaging Biol. 21, 567–573 (2019).
Sawicki, L. M. et al. Diagnostic potential of PET/CT using a 68Ga-labelled prostate-specific membrane antigen ligand in whole-body staging of renal cell carcinoma: initial experience. Eur. J. Nucl. Med. Mol. Imaging 44, 102–107 (2017).
Valls, L. et al. Early response monitoring of receptor tyrosine kinase inhibitor therapy in metastatic renal cell carcinoma using [F-18]fluorothymidine-positron emission tomography-magnetic resonance. Semin. Roentgenol. 49, 238–241 (2014).
Ukon, N. et al. Dynamic PET evaluation of elevated FLT level after sorafenib treatment in mice bearing human renal cell carcinoma xenograft. EJNMMI Res. 6, 90 (2016).
Wong, P. K. et al. In vivo imaging of cellular proliferation in renal cell carcinoma using 18F-fluorothymidine PET. Asia Ocean. J. Nucl. Med. Biol. 2, 3–11 (2014).
Stillebroer, A. B., Mulders, P. F., Boerman, O. C., Oyen, W. J. & Oosterwijk, E. Carbonic anhydrase IX in renal cell carcinoma: implications for prognosis, diagnosis, and therapy. Eur. Urol. 58, 75–83 (2010).
Pastorek, J. & Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin. Cancer Biol. 31, 52–64 (2015).
Oosterwijk, E. et al. Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J. Clin. Oncol. 11, 738–750 (1993).
Divgi, C. R. et al. Phase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal cell carcinoma. Clin. Cancer Res. 4, 2729–2739 (1998).
Brouwers, A. H. et al. 131I-cG250 monoclonal antibody immunoscintigraphy versus [18F]FDG-PET imaging in patients with metastatic renal cell carcinoma: a comparative study. Nucl. Med. Commun. 23, 229–236 (2002).
Brouwers, A. H. et al. Targeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with 131I or 111In: an intrapatient comparison. Clin. Cancer Res. 9, 3953S–3960S (2003).
Steffens, M. G. et al. Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. J. Clin. Oncol. 15, 1529–1537 (1997).
Divgi, C. R. et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J. Clin. Oncol. 31, 187–194 (2013).
Pryma, D. A. et al. Correlation of in vivo and in vitro measures of carbonic anhydrase IX antigen expression in renal masses using antibody 124I-cG250. J. Nucl. Med. 52, 535–540 (2011).
Yu, Z. et al. Anti-G250 nanobody-functionalized nanobubbles targeting renal cell carcinoma cells for ultrasound molecular imaging. Nanotechnology 31, 205101 (2020).
Turkbey, B. et al. PET/CT imaging of renal cell carcinoma with 18F-VM4-037: a phase II pilot study. Abdom. Radiol. 41, 109–118 (2016).
Garousi, J. et al. Comparative evaluation of affibody molecules for radionuclide imaging of in vivo expression of carbonic anhydrase IX. Mol. Pharm. 13, 3676–3687 (2016).
Yang, X. et al. Imaging of carbonic anhydrase IX with an 111In-labeled dual-motif inhibitor. Oncotarget 6, 33733–33742 (2015).
Minn, I. et al. [64Cu]XYIMSR-06: a dual-motif CAIX ligand for PET imaging of clear cell renal cell carcinoma. Oncotarget 7, 56471–56479 (2016).
Oosting, S. F. et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med. 56, 63–69 (2015).
van Es, S. C. et al. 89Zr-Bevacizumab PET: potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. J. Nucl. Med. 58, 905–910 (2017).
Smeenge, M. et al. First-in-human ultrasound molecular imaging with a VEGFR2-specific ultrasound molecular contrast agent (BR55) in prostate cancer: a safety and feasibility pilot study. Invest. Radiol. 52, 419–427 (2017).
Rojas, J. D. et al. Ultrasound molecular imaging of VEGFR-2 in clear-cell renal cell carcinoma tracks disease response to antiangiogenic and notch-inhibition therapy. Theranostics 8, 141–155 (2018).
Wei, S. et al. Targeted contrast-enhanced ultrasound imaging of angiogenesis in an orthotopic mouse tumor model of renal carcinoma. Ultrasound Med. Biol. 40, 1250–1259 (2014).
Mena, E. et al. [18F]fluciclatide in the in vivo evaluation of human melanoma and renal tumors expressing alphavbeta 3 and alpha vbeta 5 integrins. Eur. J. Nucl. Med. Mol. Imaging 41, 1879–1888 (2014).
Vento, J. et al. PD-L1 detection using 89Zr-atezolizumab immuno-PET in renal cell carcinoma tumorgrafts from a patient with favorable nivolumab response. J. Immunother. Cancer 7, 144 (2019).
Bensch, F. et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 24, 1852–1858 (2018).
Mao, W. et al. Intravoxel incoherent motion diffusion-weighted imaging for the assessment of renal fibrosis of chronic kidney disease: a preliminary study. Magn. Reson. Imaging 47, 118–124 (2018).
Poynton, C. B. et al. Intravoxel incoherent motion analysis of renal allograft diffusion with clinical and histopathological correlation in pediatric kidney transplant patients: a preliminary cross-sectional observational study. Pediatr. Transplant. 21, e12996 (2017).
Ong, E. et al. Modelling kidney disease using ontology: insights from the Kidney Precision Medicine Project. Nat. Rev. Nephrol. 16, 686–696 (2020).
Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 589, 281–286 (2021).
Smith, A. et al. Detecting proteomic indicators to distinguish diabetic nephropathy from hypertensive nephrosclerosis by integrating matrix-assisted laser desorption/ionization mass spectrometry imaging with high-mass accuracy mass spectrometry. Kidney Blood Press. Res. 45, 233–248 (2020).
Ivanova, M. et al. Matrix-assisted laser desorption/ionization mass spectrometry imaging to uncover protein alterations associated with the progression of IgA nephropathy. Virchows Arch. 476, 903–914 (2020).
Smith, A. et al. High spatial resolution MALDI-MS imaging in the study of membranous nephropathy. Proteom. Clin. Appl. 13, e1800016 (2019).
Smith, A. et al. α-1-Antitrypsin detected by MALDI imaging in the study of glomerulonephritis: its relevance in chronic kidney disease progression. Proteomics 16, 1759–1766 (2016).
Abdelmoula, W. M. et al. Automatic 3D nonlinear registration of mass spectrometry imaging and magnetic resonance imaging data. Anal. Chem. 91, 6206–6216 (2019).
Beck, L. H. Jr. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Rinschen, M. M. & Saez-Rodriguez, J. The tissue proteome in the multi-omic landscape of kidney disease. Nat. Rev. Nephrol. 17, 205–219 (2021).
Du, B., Yu, M. & Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 3, 358–374 (2018).
Bennett, K. M. et al. Use of cationized ferritin nanoparticles to measure renal glomerular microstructure with MRI. Methods Mol. Biol. 1397, 67–79 (2016).
Choi, C. H., Zuckerman, J. E., Webster, P. & Davis, M. E. Targeting kidney mesangium by nanoparticles of defined size. Proc. Natl Acad. Sci. USA 108, 6656–6661 (2011).
Williams, R. M. et al. Selective nanoparticle targeting of the renal tubules. Hypertension 71, 87–94 (2018).
Ordikhani, F. et al. Selective trafficking of light chain-conjugated nanoparticles to the kidney and renal cell carcinoma. Nano Today 35, 100990 (2020).
Kiessling, F., Mertens, M. E., Grimm, J. & Lammers, T. Nanoparticles for imaging: top or flop? Radiology 273, 10–28 (2014).
Ronco, C., Bellomo, R. & Kellum, J. A. Acute kidney injury. Lancet 394, 1949–1964 (2019).
Tesch, G. H. Review: serum and urine biomarkers of kidney disease: a pathophysiological perspective. Nephrology 15, 609–616 (2010).
Papasotiriou, M. et al. Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol. Dial. Transpl. 30, 1112–1121 (2015).
Genovese, F. et al. Turnover of type III collagen reflects disease severity and is associated with progression and microinflammation in patients with IgA nephropathy. Nephrol. Dial. Transpl. 31, 472–479 (2016).
Park, U. J. et al. Use of early postoperative MAG3 renal scan to predict long-term outcomes of renal transplants. Exp. Clin. Transpl. 11, 118–121 (2013).
Pijl, J. P., Kwee, T. C., Slart, R. & Glaudemans, A. FDG-PET/CT for diagnosis of cyst infection in autosomal dominant polycystic kidney disease. Clin. Transl. Imaging 6, 61–67 (2018).
Salaman, J. R. & Blandy, J. P. The use of radioactive fibrinogen as a means for detecting rejection of human renal transplants. Br. J. Surg. 57, 855 (1970).
Reuter, S. et al. Potential of noninvasive serial assessment of acute renal allograft rejection by 18F-FDG PET to monitor treatment efficiency. J. Nucl. Med. 51, 1644–1652 (2010).
Reuter, S. et al. Non-invasive imaging of acute renal allograft rejection in rats using small animal F-FDG-PET. PLoS ONE 4, e5296 (2009).
Grabner, A. et al. Non-invasive imaging of acute allograft rejection after rat renal transplantation using 18F-FDG PET. J. Vis. Exp. 28, e4240 (2013).
Acknowledgements
This work was funded by the German Research Foundation (DFG; SFB/TRR57 P25&P33, SFB/TRR219 Project-ID 322900939, BO3755/13-1 Project-ID 454024652, Research Training Group 331065168), the German Federal Ministry of Education and Research (BMBF: STOP-FSGS-01GM1901A), the German Federal Ministry of Economic Affairs and Energy (BMWi: EMPAIA project), the Medical Faculty of the RWTH Aachen (START 109/20), the European Research Council (ERC: CoG-864121 Meta-Targeting and 101001791 AIM.imaging.CKD), the ITN INTRICARE of European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska Curie (grant 722609).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to discussions of the manuscript content. B.M.K. researched the data for the article and wrote the first draft of the manuscript. T.L., F.M.M., F.K., J.F. and P.B. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.M.M. is an advisory board member for Advanced Accelerator Applications/Novartis and Bayer, holds speaker positions at Siemens, GE Healthcare and Bayer, and receives institutional grants from GE Healthcare and Nanomab. F.K. consults for Merck Darmstadt, holds patents and has running patent applications with Bayer, Roche and Visualsonics, has stock options for Molecular Targeting Inc. and co-owns InVivoContrast GmbH. The remaining authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks A. Smith, L.J. Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Nanobodies
-
Antibody fragments in the form of a single monomeric variable antibody domain; also known as single-domain antibodies.
- Aptamers
-
Molecules (DNA or RNA oligonucleotides) that bind with high affinity to a target molecule.
- Antibody- or peptide-functionalized microbubbles
-
Gas-filled microbubbles with a surface that is functionalized with antibodies or peptides as targeting ligands for molecular ultrasound imaging.
- Extraction efficiency
-
A metric used to assess the elimination of an agent from the blood by comparing its arterial and venous concentrations.
- Banff score
-
An international consensus classification for the reporting of biopsy findings from solid organ transplants.
- Standardized uptake value
-
A semi-quantitative measure commonly used in PET imaging for nuclide enrichment that takes into account nuclide decay, administered dose and patient weight.
- Mean transit time
-
The average time required for a tracer to travel through a tissue.
- Time–activity curve
-
A graph in which radioactivity is plotted against time to display the kinetics of a tracer.
Rights and permissions
About this article
Cite this article
Klinkhammer, B.M., Lammers, T., Mottaghy, F.M. et al. Non-invasive molecular imaging of kidney diseases. Nat Rev Nephrol 17, 688–703 (2021). https://doi.org/10.1038/s41581-021-00440-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00440-4
This article is cited by
-
In vivo ultrasound-induced luminescence molecular imaging
Nature Photonics (2024)
-
[68 Ga]Ga-FAPI uptake correlates with the state of chronic kidney disease
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
Homodimer 99mTc-HYNIC-E(SSSLTVPWY)2 peptide improved HER2-overexpressed tumor targeting and imaging
Medical Oncology (2022)
-
Luminescent Metal Complexes for Bioassays in the Near-Infrared (NIR) Region
Topics in Current Chemistry (2022)