Abstract
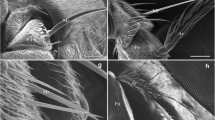
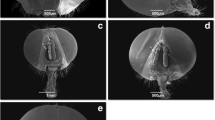
Cephalopina titillator had adverse effects on the camel health such as difficult breathing, reduction of milk production, fertility, and tissue damage. Therfore; this study aimed to describe of the fine ultrastructure of the adult fly and different stages of larvae using light and scanning electron microscopy (SEM). The larvae collected from the slaughtered camel at Cairo slaughter-house from January to May 2019. The second and third instar larvae of C. titillator were collected from the nasal passages; sinuses and turbinates. The pseudocephalon of the third larval stage had two long curved maxillae and two antennary lobes which provided several sensory papillae. However, the last abdominal segment showed two peritremes in a deep pit surrounded with two lips that contain several large sensory papillae and small spines at the tegument surface. Many basiconic sensilla are situated on the ventral depression close to the posterior spiracular. Sensilla on insect body parts play an important role in their behavior for deposited larvae. This is the first overall observation about sensilla on the whole body of Cephalopina titillator adults and larvae, which have great significance on the behavior, identification, and evolution of the adult fly.







Similar content being viewed by others
References
AbdElKader NA, Sheta E, AbuBakr HO, El-Shamy OAA, Oryan A, Attia MM (2020) Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera: Gasterophilidae) larvae in donkeys (Equus asinus). Int J Trop Insect Sci. https://doi.org/10.1007/s42690-020-00171-2
Abdelsalam M, Attia MM, Mahmoud MA (2020) Comparative morphomolecular identification and pathological changes associated with Anisakis simplex larvae (Nematoda: Anisakidae) infecting native and imported chub mackerel (Scomber japonicus) in Egypt. Regional Studies in Marine Science 39:101469. https://doi.org/10.1016/j.rsma.2020.101469
AbdElKader NA, Sheta E, AbuBakr HO, El-Shamy OAA, Oryan A, Attia MM (2020) Effects of chitosan nanoparticles, ivermectin and their combination in the treatment of Gasterophilus intestinalis (Diptera: Gasterophilidae) larvae in donkeys (Equus asinus). Int J Trop Insect Sci. https://doi.org/10.1007/s42690-020-00171-2
Abu-Elala NM, Attia MM, Abd-Elsalam RM (2018) Chitosan-silver nanocomposites in goldfish aquaria: A new perspective in Lernaea cyprinacea control. Int J Biol Macromol 111:614–622
Al-Ani F, Amr Z (2016) Seasonal prevalence of the larvae of the nasal fly (Cephalopina titillator) in camels in Jordan. Revue D’élevage Et De Médecine Vétérinaire Des Pays Tropicaux 69(3):125–127
Al Ahmed AM (2002) Seasonal prevalence of Cephalopina titillator larvae in camels in Riyadh region, Saudi Arabia. Arab Gulf J Sci Res 20(3):161–164
Al-Jindeel TJ, Jasim HJ, Alsalih NJ, Al-Yasari AMR (2018) Clinical, immunological and epidemiological studies of nasopharyngeal myiasis in camels slaughtered in al-muthanna province. Adv Anim Vet Sci 6(7):299-305
Angulo-Valadez CE, Ascencio F, Jacquiet P, Dorchies P, Cepeda-Palacios R (2011) Sheep and goat immune responses to nose bot infestation: a review. Med Vet Entomol 25(2):117–125
Attia MM, Khalifa MM, Mahdy OA (2018) The prevalence of Gasterophilus intestinalis (Diptera: Oestridae) in donkeys (Equus asinus) in Egypt with special reference to larvicidal effects of neem seed oil extract (Azadirachta indica) on third stage larvae. Open Vet J 8(4):423–431
Attia MM, Salaeh NMK (2020) Ultrastructure of adult Gasterophilus intestinalis (Diptera: Gasterophilidae) and its puparium. Int J Trop Insect Sci. https://doi.org/10.1007/s42690-019-00084-9
Attia MM, Farag HS, Abdel-Saeed H, Ismael E (2020) Advanced immunological studies on Cephalopina titillator with special references to the epidemiological uses of Dot-ELISA in camel sera. J Parasit Dis. https://doi.org/10.1007/s12639-020-01256-y
Cepeda-palacios R, Ruiz-ochoa C, Ramírez-orduña R, Angulo-Valadez CE, Dorchies P (2015) The puparium structure of the sheep nasal botfly (Oestrus ovis L.). Revue de Méd Vét 166:215–220
De Carvalho CJB, Mello-Patiu CA (2008) Key to the adults of the most common forensic species of Diptera in South America. Rev Bras Entomol 52:39-406
El-Rahman SSA (2010) Prevalence and pathology of nasal myiasis in camels slaughtered in El-Zawia Province-Western Libya: with a reference to thyroid alteration and renal lipidosis. Global Vet 4(2):190–197
Fatani A, Hilali M (1994) Prevalence and monthly variations of the second and third instars of Cephalopina titillator (Diptera: Oestridae) infesting camels(Camelus dromedarius) in the Eastern Province of Saudi Arabia. Vet Parasit 53(1/2):145–151. https://doi.org/10.1016/0304-4017(94)90026-4
Higgins A, Kock R (1984) The camel in health and disease. I. A guide to the clinical examination, chemical restraint and medication of the camel. Br Vet J 140:485–504
Hilali M, Mahdy OA, Attia MM (2015) Monthly variations of Rhinoestrus spp. (Diptera: Oestridae) larvae infesting donkeys in Egypt: Morphological and molecular identification of third stage larvae. J Adv Res 6:1015–1021
Jalali MHR, Dehghan S, Haji A (2016) Myiasis caused by Cephalopina titillator (Diptera: Oestridae) in camels (Camelus dromedarius) of semi-arid areas in Iran: distribution and associated risk factors. Comp Clin Pathol 25:677–680. https://doi.org/10.1007/s00580-016-2246-9
Kurauchi T, Nakamura T, Toh Y, Ichikawa T, Ichikawa T (2011) Distribution of mechanoreceptive sensilla and their functions in the defensive behavior of tenebrionid beetle pupae. Open Access Insect Physiology 3:13–25
Li S, Zhamg W, Wang X, Lei C, Zhu F (2016) Ultrastructure of sensilla on larvae and adults of Chrysomya megacephala (Diptera: Calliphoridae). Entomol News 126(1):52–63
Mahdy OA, Attia MM (2020) Comparative micro-morphological and phylogenetic analysis between Rhinoestrus purpureus and Rhinoestrus usbekistanicus (Diptera: Oestridae) larvae and its adults. Int J Trop Insect Sci. https://doi.org/10.1007/s42690-020-00199-4
Oryan A, Valinezhad A, Moraveji M (2008) Prevalence and pathology of camel nasal myiasis in eastern areas of Iran. Trop Biomed 25(1):30–36 (PMID: 18600202)
Otranto D (2001) The immunology of myiasis: parasite survival and host defense strategies. Trends Parasitol 17(4):176–182
Pritchard MH, Kruse GO (1982) Stains and staining methods. In the collection and preservation of animal parasites. USA: The Harold W. Manter Laboratory, University of Nebraska Press; 141
Salem HM, Attia MM (2021) Accidental intestinal myiasis caused by Musca domestica L. (Diptera:Muscidae) larvae in broiler chickens: a field study. Int J Trop Insect Sci. https://doi.org/10.1007/s42690-021-00492-w
Ramadan M (1997) Studies on some ectoparasites of camels. PhD thesis. Faculty of Veterinary Medicine, Zagazig University, Benha Branch, Egypt
Zumpt F (1965) Myiasis in Man and Animals in the Old World. Butterworth, London, pp 159–164
Funding
All authors declare that they have no funding to support this study.
Author information
Authors and Affiliations
Contributions
All authors have the same aim of the study; Marwa M. Attia Collection of the samples; Marwa M. Attia and Olfat A. Mahdy identify the parasites and apply the fine structure description of the parasite. All authors took part in writing of this manuscript and revised it.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Attia, M.M., Mahdy, O.A. Fine structure of different stages of camel nasal bot; Cephalopina titillator (Diptera: Oestridae). Int J Trop Insect Sci 42, 677–684 (2022). https://doi.org/10.1007/s42690-021-00590-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00590-9